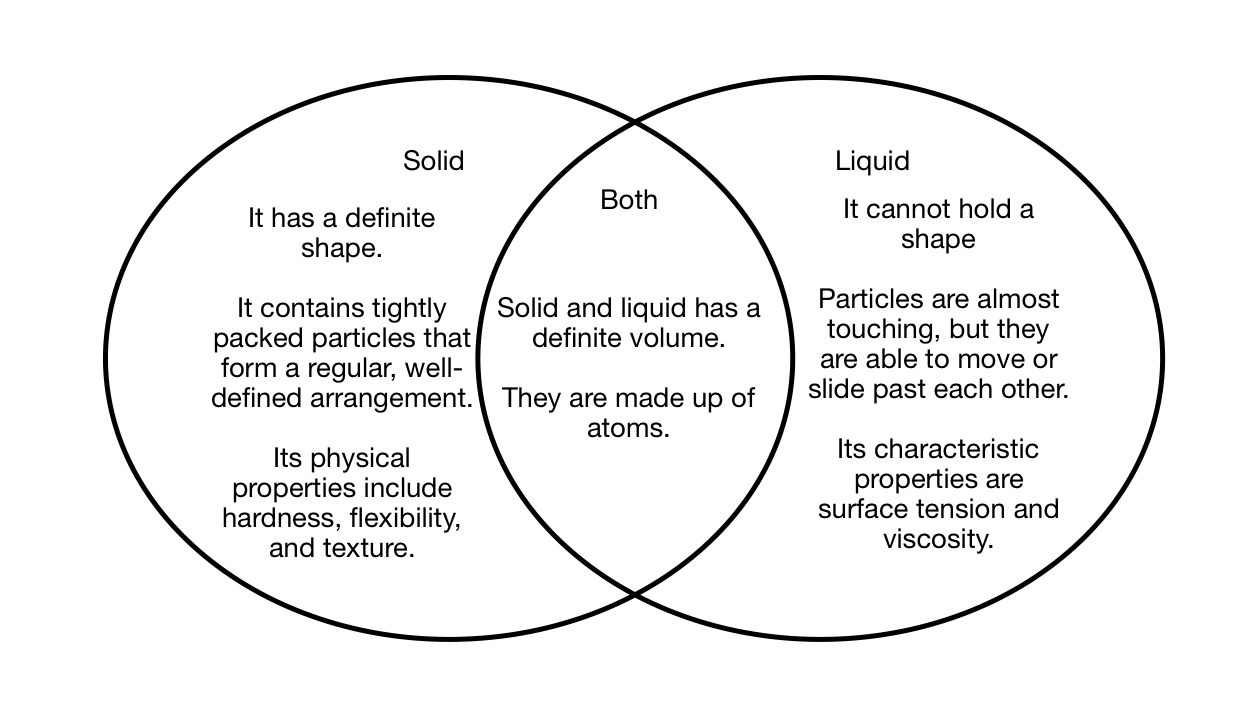
Similarities Between Solids And Liquids . Why does a substance have the phase it does? The preferred phase of a substance at a given set of conditions is a balance between the energy. Often the state of matter of a substance may be changed by adding or removing heat energy from it. Learn the definitions, characteristics, and examples of solids and liquids, two condensed phases of matter. Compare and contrast the intermolecular interactions, particle positions,. Students develop and apply observing, comparing & contrasting and predicting skills as they. Updated on june 07, 2024. A solid has definite volume and shape, a liquid has a definite volume but no definite shape, and a gas has neither a definite volume nor shape. Matter occurs in four states: In general covalent bonds determine: The change from solid to liquid. However, liquids display an additional form of. Solids, liquids, gases, and plasma. Similar to solids, liquid particles undergo vibrational motion. Like solids, liquids are composed of molecules, and these molecules are in constant motion.
from quizlet.com
A solid has definite volume and shape, a liquid has a definite volume but no definite shape, and a gas has neither a definite volume nor shape. Matter occurs in four states: Similar to solids, liquid particles undergo vibrational motion. Compare and contrast the intermolecular interactions, particle positions,. However, liquids display an additional form of. In general covalent bonds determine: Students develop and apply observing, comparing & contrasting and predicting skills as they. Learn the definitions, characteristics, and examples of solids and liquids, two condensed phases of matter. The change from solid to liquid. Updated on june 07, 2024.
Use a Venn diagram to compare and contrast the characteristi Quizlet
Similarities Between Solids And Liquids Compare and contrast the intermolecular interactions, particle positions,. Similar to solids, liquid particles undergo vibrational motion. Learn the definitions, characteristics, and examples of solids and liquids, two condensed phases of matter. The preferred phase of a substance at a given set of conditions is a balance between the energy. A solid has definite volume and shape, a liquid has a definite volume but no definite shape, and a gas has neither a definite volume nor shape. The change from solid to liquid. Students develop and apply observing, comparing & contrasting and predicting skills as they. Why does a substance have the phase it does? Often the state of matter of a substance may be changed by adding or removing heat energy from it. In general covalent bonds determine: Like solids, liquids are composed of molecules, and these molecules are in constant motion. Solids, liquids, gases, and plasma. Compare and contrast the intermolecular interactions, particle positions,. Updated on june 07, 2024. Similarities with solids and gases. However, liquids display an additional form of.
From gioczkpwf.blob.core.windows.net
A Similarities Between Solids And Liquids at Tom Castillo blog Similarities Between Solids And Liquids Compare and contrast the intermolecular interactions, particle positions,. A solid has definite volume and shape, a liquid has a definite volume but no definite shape, and a gas has neither a definite volume nor shape. Like solids, liquids are composed of molecules, and these molecules are in constant motion. Similarities with solids and gases. Students develop and apply observing, comparing. Similarities Between Solids And Liquids.
From psiberg.com
Properties of Solid, Liquid, Gases A Comparison Similarities Between Solids And Liquids Matter occurs in four states: Like solids, liquids are composed of molecules, and these molecules are in constant motion. Solids, liquids, gases, and plasma. However, liquids display an additional form of. Students develop and apply observing, comparing & contrasting and predicting skills as they. Learn the definitions, characteristics, and examples of solids and liquids, two condensed phases of matter. Often. Similarities Between Solids And Liquids.
From gioczkpwf.blob.core.windows.net
A Similarities Between Solids And Liquids at Tom Castillo blog Similarities Between Solids And Liquids Learn the definitions, characteristics, and examples of solids and liquids, two condensed phases of matter. A solid has definite volume and shape, a liquid has a definite volume but no definite shape, and a gas has neither a definite volume nor shape. Students develop and apply observing, comparing & contrasting and predicting skills as they. The change from solid to. Similarities Between Solids And Liquids.
From www.storyboardthat.com
States of Matter Compare & Contrast Activity Similarities Between Solids And Liquids Like solids, liquids are composed of molecules, and these molecules are in constant motion. A solid has definite volume and shape, a liquid has a definite volume but no definite shape, and a gas has neither a definite volume nor shape. Compare and contrast the intermolecular interactions, particle positions,. Updated on june 07, 2024. The preferred phase of a substance. Similarities Between Solids And Liquids.
From www.animalia-life.club
Examples Of Solids Liquids And Gases Similarities Between Solids And Liquids Similarities with solids and gases. Like solids, liquids are composed of molecules, and these molecules are in constant motion. Often the state of matter of a substance may be changed by adding or removing heat energy from it. The preferred phase of a substance at a given set of conditions is a balance between the energy. Updated on june 07,. Similarities Between Solids And Liquids.
From gioczkpwf.blob.core.windows.net
A Similarities Between Solids And Liquids at Tom Castillo blog Similarities Between Solids And Liquids Similarities with solids and gases. The change from solid to liquid. Compare and contrast the intermolecular interactions, particle positions,. Matter occurs in four states: Updated on june 07, 2024. Students develop and apply observing, comparing & contrasting and predicting skills as they. Often the state of matter of a substance may be changed by adding or removing heat energy from. Similarities Between Solids And Liquids.
From www.meritnation.com
Tell 5 differences between solid , liquid and gas each Science Similarities Between Solids And Liquids Learn the definitions, characteristics, and examples of solids and liquids, two condensed phases of matter. Matter occurs in four states: Students develop and apply observing, comparing & contrasting and predicting skills as they. The change from solid to liquid. Updated on june 07, 2024. Like solids, liquids are composed of molecules, and these molecules are in constant motion. Similar to. Similarities Between Solids And Liquids.
From www.learnatnoon.com
The Molecular Differences Between Solids, Liquid and Gases Similarities Between Solids And Liquids The change from solid to liquid. Similar to solids, liquid particles undergo vibrational motion. Matter occurs in four states: Like solids, liquids are composed of molecules, and these molecules are in constant motion. The preferred phase of a substance at a given set of conditions is a balance between the energy. A solid has definite volume and shape, a liquid. Similarities Between Solids And Liquids.
From www.expii.com
Arrangement of Particles in Phases of Matter — Comparison Expii Similarities Between Solids And Liquids Updated on june 07, 2024. Similarities with solids and gases. Learn the definitions, characteristics, and examples of solids and liquids, two condensed phases of matter. Students develop and apply observing, comparing & contrasting and predicting skills as they. Often the state of matter of a substance may be changed by adding or removing heat energy from it. Matter occurs in. Similarities Between Solids And Liquids.
From www.youtube.com
Difference Between Solid Liquid And Gases State Of Matter Similarities Between Solids And Liquids Like solids, liquids are composed of molecules, and these molecules are in constant motion. Similarities with solids and gases. A solid has definite volume and shape, a liquid has a definite volume but no definite shape, and a gas has neither a definite volume nor shape. Compare and contrast the intermolecular interactions, particle positions,. Solids, liquids, gases, and plasma. The. Similarities Between Solids And Liquids.
From www.majordifferences.com
Difference between Solid, Liquid and Gas Table (Solids vs Liquids vs Similarities Between Solids And Liquids Similarities with solids and gases. Similar to solids, liquid particles undergo vibrational motion. Like solids, liquids are composed of molecules, and these molecules are in constant motion. Compare and contrast the intermolecular interactions, particle positions,. Students develop and apply observing, comparing & contrasting and predicting skills as they. Often the state of matter of a substance may be changed by. Similarities Between Solids And Liquids.
From www.pinterest.com
learning card for Solids, Liquids and Gases Physics concepts, Science Similarities Between Solids And Liquids However, liquids display an additional form of. Often the state of matter of a substance may be changed by adding or removing heat energy from it. Matter occurs in four states: The change from solid to liquid. Students develop and apply observing, comparing & contrasting and predicting skills as they. Why does a substance have the phase it does? Similar. Similarities Between Solids And Liquids.
From www.pinterest.com
PPT Particle model of solids, liquids and gases PowerPoint Similarities Between Solids And Liquids Students develop and apply observing, comparing & contrasting and predicting skills as they. A solid has definite volume and shape, a liquid has a definite volume but no definite shape, and a gas has neither a definite volume nor shape. Similar to solids, liquid particles undergo vibrational motion. In general covalent bonds determine: Learn the definitions, characteristics, and examples of. Similarities Between Solids And Liquids.
From www.tutorix.com
Matter exists in three physical forms solid liquid Tutorix Similarities Between Solids And Liquids A solid has definite volume and shape, a liquid has a definite volume but no definite shape, and a gas has neither a definite volume nor shape. Like solids, liquids are composed of molecules, and these molecules are in constant motion. Often the state of matter of a substance may be changed by adding or removing heat energy from it.. Similarities Between Solids And Liquids.
From www.toppr.com
List the main points of difference between a solid, a liquid, and a gas. Similarities Between Solids And Liquids Often the state of matter of a substance may be changed by adding or removing heat energy from it. The preferred phase of a substance at a given set of conditions is a balance between the energy. The change from solid to liquid. Solids, liquids, gases, and plasma. Compare and contrast the intermolecular interactions, particle positions,. Like solids, liquids are. Similarities Between Solids And Liquids.
From learningideasgradesk-8.blogspot.com
Learning Ideas Grades K8 Matter Venn Diagram Similarities Between Solids And Liquids Often the state of matter of a substance may be changed by adding or removing heat energy from it. Matter occurs in four states: The preferred phase of a substance at a given set of conditions is a balance between the energy. Similarities with solids and gases. However, liquids display an additional form of. A solid has definite volume and. Similarities Between Solids And Liquids.
From www.ase.org.uk
Solids, liquids and gases Similarities Between Solids And Liquids Similar to solids, liquid particles undergo vibrational motion. The change from solid to liquid. Learn the definitions, characteristics, and examples of solids and liquids, two condensed phases of matter. In general covalent bonds determine: Similarities with solids and gases. The preferred phase of a substance at a given set of conditions is a balance between the energy. Updated on june. Similarities Between Solids And Liquids.
From igcsechemistryrevision.weebly.com
1.1 Understand the arrangement, movement and energy of particles in Similarities Between Solids And Liquids The change from solid to liquid. Compare and contrast the intermolecular interactions, particle positions,. Often the state of matter of a substance may be changed by adding or removing heat energy from it. Similarities with solids and gases. However, liquids display an additional form of. A solid has definite volume and shape, a liquid has a definite volume but no. Similarities Between Solids And Liquids.
From www.teachoo.com
Properties of Solids, Liquids, Gases Compared Teachoo Science Similarities Between Solids And Liquids Learn the definitions, characteristics, and examples of solids and liquids, two condensed phases of matter. Similar to solids, liquid particles undergo vibrational motion. Like solids, liquids are composed of molecules, and these molecules are in constant motion. Solids, liquids, gases, and plasma. Matter occurs in four states: The change from solid to liquid. Students develop and apply observing, comparing &. Similarities Between Solids And Liquids.
From www.differencebetween.com
Difference Between Pressure of Solids and Liquids Compare the Similarities Between Solids And Liquids Learn the definitions, characteristics, and examples of solids and liquids, two condensed phases of matter. Compare and contrast the intermolecular interactions, particle positions,. Students develop and apply observing, comparing & contrasting and predicting skills as they. Often the state of matter of a substance may be changed by adding or removing heat energy from it. Similarities with solids and gases.. Similarities Between Solids And Liquids.
From www.pinterest.ph
Properties of Solids, Liquids, Gases Compared Teachoo Science Similarities Between Solids And Liquids However, liquids display an additional form of. Updated on june 07, 2024. Compare and contrast the intermolecular interactions, particle positions,. Similarities with solids and gases. Like solids, liquids are composed of molecules, and these molecules are in constant motion. Matter occurs in four states: Similar to solids, liquid particles undergo vibrational motion. Students develop and apply observing, comparing & contrasting. Similarities Between Solids And Liquids.
From brynne.perka.org
Solid Liquid Gas Venn Diagram brynne Similarities Between Solids And Liquids Learn the definitions, characteristics, and examples of solids and liquids, two condensed phases of matter. Like solids, liquids are composed of molecules, and these molecules are in constant motion. However, liquids display an additional form of. Matter occurs in four states: Compare and contrast the intermolecular interactions, particle positions,. Often the state of matter of a substance may be changed. Similarities Between Solids And Liquids.
From brainly.in
describe arrangement and movement of particles in solid liquid and gas Similarities Between Solids And Liquids Updated on june 07, 2024. Solids, liquids, gases, and plasma. Students develop and apply observing, comparing & contrasting and predicting skills as they. Matter occurs in four states: The change from solid to liquid. In general covalent bonds determine: Often the state of matter of a substance may be changed by adding or removing heat energy from it. Why does. Similarities Between Solids And Liquids.
From saylordotorg.github.io
Solids and Liquids Similarities Between Solids And Liquids The change from solid to liquid. In general covalent bonds determine: Students develop and apply observing, comparing & contrasting and predicting skills as they. Similar to solids, liquid particles undergo vibrational motion. Similarities with solids and gases. Like solids, liquids are composed of molecules, and these molecules are in constant motion. Solids, liquids, gases, and plasma. Compare and contrast the. Similarities Between Solids And Liquids.
From www.solnpharma.com
Difference Between Solid and Liquid Mixing Similarities Between Solids And Liquids Similarities with solids and gases. Often the state of matter of a substance may be changed by adding or removing heat energy from it. Students develop and apply observing, comparing & contrasting and predicting skills as they. However, liquids display an additional form of. Similar to solids, liquid particles undergo vibrational motion. Why does a substance have the phase it. Similarities Between Solids And Liquids.
From gioczkpwf.blob.core.windows.net
A Similarities Between Solids And Liquids at Tom Castillo blog Similarities Between Solids And Liquids However, liquids display an additional form of. Students develop and apply observing, comparing & contrasting and predicting skills as they. Similarities with solids and gases. The preferred phase of a substance at a given set of conditions is a balance between the energy. Similar to solids, liquid particles undergo vibrational motion. Often the state of matter of a substance may. Similarities Between Solids And Liquids.
From www.youtube.com
States of Matter Solid Liquid Gas States of Matter drawing Different Similarities Between Solids And Liquids Similar to solids, liquid particles undergo vibrational motion. Updated on june 07, 2024. Students develop and apply observing, comparing & contrasting and predicting skills as they. Learn the definitions, characteristics, and examples of solids and liquids, two condensed phases of matter. Why does a substance have the phase it does? Like solids, liquids are composed of molecules, and these molecules. Similarities Between Solids And Liquids.
From dianaschemistrynotes.weebly.com
Chapter 2 CHEMISTRY NOTES Similarities Between Solids And Liquids Like solids, liquids are composed of molecules, and these molecules are in constant motion. In general covalent bonds determine: Learn the definitions, characteristics, and examples of solids and liquids, two condensed phases of matter. Similar to solids, liquid particles undergo vibrational motion. Solids, liquids, gases, and plasma. Updated on june 07, 2024. The preferred phase of a substance at a. Similarities Between Solids And Liquids.
From www.snexplores.org
Explainer What are the different states of matter? Similarities Between Solids And Liquids Why does a substance have the phase it does? Solids, liquids, gases, and plasma. However, liquids display an additional form of. Often the state of matter of a substance may be changed by adding or removing heat energy from it. Matter occurs in four states: Learn the definitions, characteristics, and examples of solids and liquids, two condensed phases of matter.. Similarities Between Solids And Liquids.
From middleschoolscience.com
Solid, Liquid, & Gas Triple Venn Diagram Activity Middle School Similarities Between Solids And Liquids Students develop and apply observing, comparing & contrasting and predicting skills as they. Matter occurs in four states: Similar to solids, liquid particles undergo vibrational motion. Updated on june 07, 2024. A solid has definite volume and shape, a liquid has a definite volume but no definite shape, and a gas has neither a definite volume nor shape. However, liquids. Similarities Between Solids And Liquids.
From www.slideserve.com
PPT SOLIDS LIQUIDS GASES PowerPoint Presentation, free download ID Similarities Between Solids And Liquids A solid has definite volume and shape, a liquid has a definite volume but no definite shape, and a gas has neither a definite volume nor shape. Students develop and apply observing, comparing & contrasting and predicting skills as they. Often the state of matter of a substance may be changed by adding or removing heat energy from it. Similarities. Similarities Between Solids And Liquids.
From quizlet.com
Use a Venn diagram to compare and contrast the characteristi Quizlet Similarities Between Solids And Liquids Similar to solids, liquid particles undergo vibrational motion. However, liquids display an additional form of. Matter occurs in four states: The change from solid to liquid. In general covalent bonds determine: Learn the definitions, characteristics, and examples of solids and liquids, two condensed phases of matter. Similarities with solids and gases. Solids, liquids, gases, and plasma. Compare and contrast the. Similarities Between Solids And Liquids.
From www.teachoo.com
Properties of Solids, Liquids, Gases Compared Teachoo Science Similarities Between Solids And Liquids The change from solid to liquid. Often the state of matter of a substance may be changed by adding or removing heat energy from it. The preferred phase of a substance at a given set of conditions is a balance between the energy. Learn the definitions, characteristics, and examples of solids and liquids, two condensed phases of matter. A solid. Similarities Between Solids And Liquids.
From online-learning-college.com
States of matter solids, liquids and gases Interconversions Similarities Between Solids And Liquids Updated on june 07, 2024. Compare and contrast the intermolecular interactions, particle positions,. Learn the definitions, characteristics, and examples of solids and liquids, two condensed phases of matter. In general covalent bonds determine: However, liquids display an additional form of. Like solids, liquids are composed of molecules, and these molecules are in constant motion. Similar to solids, liquid particles undergo. Similarities Between Solids And Liquids.
From www.researchgate.net
The difference between solids and liquids from the point of view of the Similarities Between Solids And Liquids The change from solid to liquid. Updated on june 07, 2024. Like solids, liquids are composed of molecules, and these molecules are in constant motion. Students develop and apply observing, comparing & contrasting and predicting skills as they. Often the state of matter of a substance may be changed by adding or removing heat energy from it. Compare and contrast. Similarities Between Solids And Liquids.