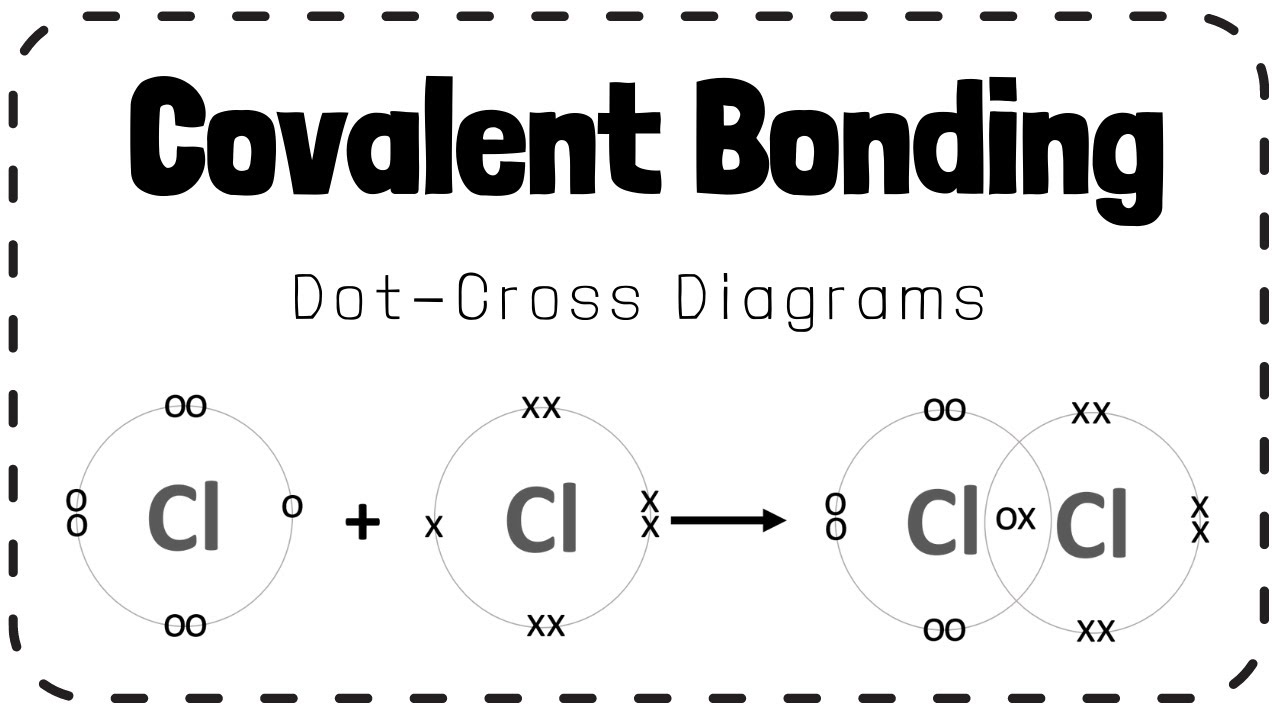
Chlorine Dot Cross Diagram . Representing dot & cross diagrams. Covalent substances tend to have simple molecular structures, such as cl 2, h 2 o or co. Dot and cross diagrams for covalent compounds. Add dots to represent the outer electrons of one type of atom (h). Only the outer electrons are shown. The electrons are shown as dots and crosses. Here’s an example using sodium and chlorine. (lewis diagram of chlorine on the left) simplified 'dot and cross' electronic diagram for. Since chlorine is found in group 7a of the periodic table, it contains 7 valence electrons. Add crosses to represent the outer electrons of the other type of atom (cl). In a dot and cross diagram: Instead of trying to remember lots of different dot and cross diagrams, it may help to understand how to draw them. Dot and cross diagrams help us to model when ions are formed from atoms.
from diagramlibraryverb.z13.web.core.windows.net
In a dot and cross diagram: Covalent substances tend to have simple molecular structures, such as cl 2, h 2 o or co. The electrons are shown as dots and crosses. Only the outer electrons are shown. Add crosses to represent the outer electrons of the other type of atom (cl). Dot and cross diagrams help us to model when ions are formed from atoms. Since chlorine is found in group 7a of the periodic table, it contains 7 valence electrons. Representing dot & cross diagrams. Add dots to represent the outer electrons of one type of atom (h). (lewis diagram of chlorine on the left) simplified 'dot and cross' electronic diagram for.
Dot And Cross Diagram For Hydrogen Chloride
Chlorine Dot Cross Diagram The electrons are shown as dots and crosses. Dot and cross diagrams help us to model when ions are formed from atoms. The electrons are shown as dots and crosses. (lewis diagram of chlorine on the left) simplified 'dot and cross' electronic diagram for. Only the outer electrons are shown. Add dots to represent the outer electrons of one type of atom (h). Dot and cross diagrams for covalent compounds. Here’s an example using sodium and chlorine. Since chlorine is found in group 7a of the periodic table, it contains 7 valence electrons. Add crosses to represent the outer electrons of the other type of atom (cl). Instead of trying to remember lots of different dot and cross diagrams, it may help to understand how to draw them. Representing dot & cross diagrams. Covalent substances tend to have simple molecular structures, such as cl 2, h 2 o or co. In a dot and cross diagram:
From valenceelectrons.com
How to Find the Valence Electrons for ClF3 (Chlorine Trifluoride)? Chlorine Dot Cross Diagram Covalent substances tend to have simple molecular structures, such as cl 2, h 2 o or co. Representing dot & cross diagrams. In a dot and cross diagram: Add dots to represent the outer electrons of one type of atom (h). Instead of trying to remember lots of different dot and cross diagrams, it may help to understand how to. Chlorine Dot Cross Diagram.
From animalia-life.club
Lewis Dot Structure For Chlorine Chlorine Dot Cross Diagram Dot and cross diagrams for covalent compounds. Instead of trying to remember lots of different dot and cross diagrams, it may help to understand how to draw them. In a dot and cross diagram: Since chlorine is found in group 7a of the periodic table, it contains 7 valence electrons. Representing dot & cross diagrams. The electrons are shown as. Chlorine Dot Cross Diagram.
From www.linstitute.net
Edexcel IGCSE Chemistry 复习笔记 1.6 4 Ionic Bonds Dot & Cross Diagrams翰林国际教育 Chlorine Dot Cross Diagram Covalent substances tend to have simple molecular structures, such as cl 2, h 2 o or co. Add crosses to represent the outer electrons of the other type of atom (cl). Here’s an example using sodium and chlorine. Only the outer electrons are shown. Add dots to represent the outer electrons of one type of atom (h). The electrons are. Chlorine Dot Cross Diagram.
From animalia-life.club
Lewis Dot Structure For Chlorine Chlorine Dot Cross Diagram (lewis diagram of chlorine on the left) simplified 'dot and cross' electronic diagram for. The electrons are shown as dots and crosses. Covalent substances tend to have simple molecular structures, such as cl 2, h 2 o or co. Here’s an example using sodium and chlorine. Only the outer electrons are shown. Representing dot & cross diagrams. Since chlorine is. Chlorine Dot Cross Diagram.
From animalia-life.club
Lewis Dot Structure For Chlorine Chlorine Dot Cross Diagram The electrons are shown as dots and crosses. Dot and cross diagrams for covalent compounds. Representing dot & cross diagrams. Only the outer electrons are shown. In a dot and cross diagram: Add crosses to represent the outer electrons of the other type of atom (cl). Dot and cross diagrams help us to model when ions are formed from atoms.. Chlorine Dot Cross Diagram.
From www.animalia-life.club
Electron Configuration For Chlorine Chlorine Dot Cross Diagram Covalent substances tend to have simple molecular structures, such as cl 2, h 2 o or co. (lewis diagram of chlorine on the left) simplified 'dot and cross' electronic diagram for. Add dots to represent the outer electrons of one type of atom (h). Since chlorine is found in group 7a of the periodic table, it contains 7 valence electrons.. Chlorine Dot Cross Diagram.
From diagramlibraryverb.z13.web.core.windows.net
Dot And Cross Diagram For Hydrogen Chloride Chlorine Dot Cross Diagram Add crosses to represent the outer electrons of the other type of atom (cl). Add dots to represent the outer electrons of one type of atom (h). Dot and cross diagrams for covalent compounds. Only the outer electrons are shown. Dot and cross diagrams help us to model when ions are formed from atoms. In a dot and cross diagram:. Chlorine Dot Cross Diagram.
From animalia-life.club
Lewis Dot Structure For Chlorine Chlorine Dot Cross Diagram Since chlorine is found in group 7a of the periodic table, it contains 7 valence electrons. Dot and cross diagrams help us to model when ions are formed from atoms. In a dot and cross diagram: (lewis diagram of chlorine on the left) simplified 'dot and cross' electronic diagram for. Covalent substances tend to have simple molecular structures, such as. Chlorine Dot Cross Diagram.
From schematicpartebriose.z14.web.core.windows.net
Dot And Cross Diagram Of Chlorine Chlorine Dot Cross Diagram The electrons are shown as dots and crosses. Only the outer electrons are shown. Representing dot & cross diagrams. Here’s an example using sodium and chlorine. (lewis diagram of chlorine on the left) simplified 'dot and cross' electronic diagram for. Covalent substances tend to have simple molecular structures, such as cl 2, h 2 o or co. Add dots to. Chlorine Dot Cross Diagram.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 1.46 Understand How to Use DotandCross Chlorine Dot Cross Diagram Since chlorine is found in group 7a of the periodic table, it contains 7 valence electrons. Dot and cross diagrams help us to model when ions are formed from atoms. In a dot and cross diagram: Add crosses to represent the outer electrons of the other type of atom (cl). Instead of trying to remember lots of different dot and. Chlorine Dot Cross Diagram.
From animalia-life.club
Lewis Dot Structure For Chlorine Chlorine Dot Cross Diagram Here’s an example using sodium and chlorine. (lewis diagram of chlorine on the left) simplified 'dot and cross' electronic diagram for. Representing dot & cross diagrams. Since chlorine is found in group 7a of the periodic table, it contains 7 valence electrons. Dot and cross diagrams help us to model when ions are formed from atoms. In a dot and. Chlorine Dot Cross Diagram.
From guidemanualephemerist.z14.web.core.windows.net
Chlorine Dot And Cross Diagram Chlorine Dot Cross Diagram The electrons are shown as dots and crosses. Dot and cross diagrams for covalent compounds. In a dot and cross diagram: Since chlorine is found in group 7a of the periodic table, it contains 7 valence electrons. Dot and cross diagrams help us to model when ions are formed from atoms. (lewis diagram of chlorine on the left) simplified 'dot. Chlorine Dot Cross Diagram.
From diagramio.com
Chlorine dot diagram understanding its electron configuration Chlorine Dot Cross Diagram Dot and cross diagrams for covalent compounds. Add dots to represent the outer electrons of one type of atom (h). The electrons are shown as dots and crosses. Dot and cross diagrams help us to model when ions are formed from atoms. Instead of trying to remember lots of different dot and cross diagrams, it may help to understand how. Chlorine Dot Cross Diagram.
From www.alamy.com
Dot and cross diagrams for simple covalent molecules of chlorine (Cl2 Chlorine Dot Cross Diagram Since chlorine is found in group 7a of the periodic table, it contains 7 valence electrons. Dot and cross diagrams help us to model when ions are formed from atoms. Here’s an example using sodium and chlorine. The electrons are shown as dots and crosses. Add dots to represent the outer electrons of one type of atom (h). Instead of. Chlorine Dot Cross Diagram.
From wiringdatabaseinfo.blogspot.com
Lewis Dot Diagram For Chlorine Wiring Site Resource Chlorine Dot Cross Diagram Add crosses to represent the outer electrons of the other type of atom (cl). Here’s an example using sodium and chlorine. Representing dot & cross diagrams. Instead of trying to remember lots of different dot and cross diagrams, it may help to understand how to draw them. In a dot and cross diagram: Since chlorine is found in group 7a. Chlorine Dot Cross Diagram.
From kdi-ppi.com
Why Chlorine Dot Diagrams Are Essential for Understanding Chemical Bonding Chlorine Dot Cross Diagram Dot and cross diagrams for covalent compounds. Dot and cross diagrams help us to model when ions are formed from atoms. In a dot and cross diagram: (lewis diagram of chlorine on the left) simplified 'dot and cross' electronic diagram for. Since chlorine is found in group 7a of the periodic table, it contains 7 valence electrons. Add crosses to. Chlorine Dot Cross Diagram.
From guidelibcombusting.z13.web.core.windows.net
Dot And Cross Diagram Of Chlorine Chlorine Dot Cross Diagram Add dots to represent the outer electrons of one type of atom (h). Representing dot & cross diagrams. Covalent substances tend to have simple molecular structures, such as cl 2, h 2 o or co. Only the outer electrons are shown. Instead of trying to remember lots of different dot and cross diagrams, it may help to understand how to. Chlorine Dot Cross Diagram.
From www.vectorstock.com
Diagram representation of the element chlorine Vector Image Chlorine Dot Cross Diagram Dot and cross diagrams for covalent compounds. In a dot and cross diagram: (lewis diagram of chlorine on the left) simplified 'dot and cross' electronic diagram for. Covalent substances tend to have simple molecular structures, such as cl 2, h 2 o or co. Only the outer electrons are shown. Dot and cross diagrams help us to model when ions. Chlorine Dot Cross Diagram.
From www.thesciencehive.co.uk
Covalent bonding (GCSE) — the science sauce Chlorine Dot Cross Diagram (lewis diagram of chlorine on the left) simplified 'dot and cross' electronic diagram for. Covalent substances tend to have simple molecular structures, such as cl 2, h 2 o or co. Add crosses to represent the outer electrons of the other type of atom (cl). Only the outer electrons are shown. Since chlorine is found in group 7a of the. Chlorine Dot Cross Diagram.
From animalia-life.club
Lewis Dot Structure For Chlorine Chlorine Dot Cross Diagram Representing dot & cross diagrams. Instead of trying to remember lots of different dot and cross diagrams, it may help to understand how to draw them. In a dot and cross diagram: Covalent substances tend to have simple molecular structures, such as cl 2, h 2 o or co. Dot and cross diagrams for covalent compounds. Here’s an example using. Chlorine Dot Cross Diagram.
From www.thesciencehive.co.uk
Bonding and Structure* — the science sauce Chlorine Dot Cross Diagram Add dots to represent the outer electrons of one type of atom (h). Dot and cross diagrams for covalent compounds. Instead of trying to remember lots of different dot and cross diagrams, it may help to understand how to draw them. Covalent substances tend to have simple molecular structures, such as cl 2, h 2 o or co. Add crosses. Chlorine Dot Cross Diagram.
From chemnotcheem.com
Dot and cross diagrams of simple molecules Exam Questions Chlorine Dot Cross Diagram (lewis diagram of chlorine on the left) simplified 'dot and cross' electronic diagram for. Covalent substances tend to have simple molecular structures, such as cl 2, h 2 o or co. The electrons are shown as dots and crosses. Dot and cross diagrams help us to model when ions are formed from atoms. In a dot and cross diagram: Add. Chlorine Dot Cross Diagram.
From stock.adobe.com
Dot and cross diagrams for simple covalent molecules of chlorine (Cl2 Chlorine Dot Cross Diagram Dot and cross diagrams help us to model when ions are formed from atoms. Here’s an example using sodium and chlorine. Since chlorine is found in group 7a of the periodic table, it contains 7 valence electrons. Dot and cross diagrams for covalent compounds. (lewis diagram of chlorine on the left) simplified 'dot and cross' electronic diagram for. Covalent substances. Chlorine Dot Cross Diagram.
From www.linstitute.net
Edexcel IGCSE Chemistry 复习笔记 1.7.2 Covalent Bonds Dot & Cross Diagrams Chlorine Dot Cross Diagram Add dots to represent the outer electrons of one type of atom (h). Since chlorine is found in group 7a of the periodic table, it contains 7 valence electrons. Dot and cross diagrams help us to model when ions are formed from atoms. Dot and cross diagrams for covalent compounds. Only the outer electrons are shown. Here’s an example using. Chlorine Dot Cross Diagram.
From manuallistcantabank.z21.web.core.windows.net
Electron Dot Diagram For Chlorine Chlorine Dot Cross Diagram Since chlorine is found in group 7a of the periodic table, it contains 7 valence electrons. Covalent substances tend to have simple molecular structures, such as cl 2, h 2 o or co. In a dot and cross diagram: Instead of trying to remember lots of different dot and cross diagrams, it may help to understand how to draw them.. Chlorine Dot Cross Diagram.
From www.youtube.com
ClF3 Lewis Structure (Chlorine Trifluoride) YouTube Chlorine Dot Cross Diagram Instead of trying to remember lots of different dot and cross diagrams, it may help to understand how to draw them. Only the outer electrons are shown. Covalent substances tend to have simple molecular structures, such as cl 2, h 2 o or co. In a dot and cross diagram: Representing dot & cross diagrams. Since chlorine is found in. Chlorine Dot Cross Diagram.
From animalia-life.club
Lewis Dot Structure For Chlorine Chlorine Dot Cross Diagram Instead of trying to remember lots of different dot and cross diagrams, it may help to understand how to draw them. Representing dot & cross diagrams. Here’s an example using sodium and chlorine. Add crosses to represent the outer electrons of the other type of atom (cl). Dot and cross diagrams for covalent compounds. (lewis diagram of chlorine on the. Chlorine Dot Cross Diagram.
From wiringguidefrosts.z19.web.core.windows.net
Chlorine Dot And Cross Diagram Chlorine Dot Cross Diagram Since chlorine is found in group 7a of the periodic table, it contains 7 valence electrons. The electrons are shown as dots and crosses. Dot and cross diagrams help us to model when ions are formed from atoms. Instead of trying to remember lots of different dot and cross diagrams, it may help to understand how to draw them. Here’s. Chlorine Dot Cross Diagram.
From animalia-life.club
Potassium Chloride Shell Model Chlorine Dot Cross Diagram Covalent substances tend to have simple molecular structures, such as cl 2, h 2 o or co. Only the outer electrons are shown. Dot and cross diagrams for covalent compounds. Dot and cross diagrams help us to model when ions are formed from atoms. Add dots to represent the outer electrons of one type of atom (h). (lewis diagram of. Chlorine Dot Cross Diagram.
From enginewiringlee.z13.web.core.windows.net
Dot And Cross Diagram For Sodium Chloride Chlorine Dot Cross Diagram Instead of trying to remember lots of different dot and cross diagrams, it may help to understand how to draw them. Dot and cross diagrams help us to model when ions are formed from atoms. Representing dot & cross diagrams. Dot and cross diagrams for covalent compounds. Since chlorine is found in group 7a of the periodic table, it contains. Chlorine Dot Cross Diagram.
From www.ck12.org
Molecular Geometry CK12 Foundation Chlorine Dot Cross Diagram Since chlorine is found in group 7a of the periodic table, it contains 7 valence electrons. The electrons are shown as dots and crosses. Add dots to represent the outer electrons of one type of atom (h). Dot and cross diagrams help us to model when ions are formed from atoms. Representing dot & cross diagrams. (lewis diagram of chlorine. Chlorine Dot Cross Diagram.
From www.slideserve.com
PPT AS Chemistry PowerPoint Presentation, free download ID2093824 Chlorine Dot Cross Diagram The electrons are shown as dots and crosses. Here’s an example using sodium and chlorine. Add dots to represent the outer electrons of one type of atom (h). Add crosses to represent the outer electrons of the other type of atom (cl). Since chlorine is found in group 7a of the periodic table, it contains 7 valence electrons. In a. Chlorine Dot Cross Diagram.
From diagramlibraryverb.z13.web.core.windows.net
Dot And Cross Diagram For Hydrogen Chloride Chlorine Dot Cross Diagram (lewis diagram of chlorine on the left) simplified 'dot and cross' electronic diagram for. In a dot and cross diagram: Instead of trying to remember lots of different dot and cross diagrams, it may help to understand how to draw them. Since chlorine is found in group 7a of the periodic table, it contains 7 valence electrons. The electrons are. Chlorine Dot Cross Diagram.
From animalia-life.club
Lewis Dot Structure For Chlorine Chlorine Dot Cross Diagram The electrons are shown as dots and crosses. Add dots to represent the outer electrons of one type of atom (h). Dot and cross diagrams help us to model when ions are formed from atoms. Since chlorine is found in group 7a of the periodic table, it contains 7 valence electrons. Covalent substances tend to have simple molecular structures, such. Chlorine Dot Cross Diagram.
From chemnotcheem.com
2019 chemical bonding TYS questions Chem Not Cheem Chlorine Dot Cross Diagram Add crosses to represent the outer electrons of the other type of atom (cl). In a dot and cross diagram: Dot and cross diagrams help us to model when ions are formed from atoms. Only the outer electrons are shown. Representing dot & cross diagrams. Dot and cross diagrams for covalent compounds. Here’s an example using sodium and chlorine. Instead. Chlorine Dot Cross Diagram.