Bromine Sulphur Dioxide . The oxidation of sulphur dioxide by bromine and water. The bromide ions reduce the sulphuric acid to sulphur dioxide gas. Bromide reduces sulfuric acid to sulfur dioxide gas, decreasing the oxidation state of sulfur from +6 to +4. This is a decrease of oxidation state of the sulphur from +6 in the sulphuric acid to +4 in the sulphur dioxide. Sulfur dioxide is fairly soluble in water, reacting to. Sulfur dioxide, so 2, and sulfur trioxide, so 3. The reaction of so 2, br 2 and h 2 o, forming hbr and h 2 so 4, is one of the reactions of the hybrid cycle for. See diagrams, equations, and examples of. One mole of sulfur dioxide [so 2], two moles of dibromine [br 2] and two moles of water [h 2 o] react to form four moles of bromine [br], one. Learn how halide ions can act as reducing agents, identify them with silver nitrate and ammonia, and react with concentrated sulfuric acid.
from stock.adobe.com
Learn how halide ions can act as reducing agents, identify them with silver nitrate and ammonia, and react with concentrated sulfuric acid. The reaction of so 2, br 2 and h 2 o, forming hbr and h 2 so 4, is one of the reactions of the hybrid cycle for. The oxidation of sulphur dioxide by bromine and water. One mole of sulfur dioxide [so 2], two moles of dibromine [br 2] and two moles of water [h 2 o] react to form four moles of bromine [br], one. See diagrams, equations, and examples of. Bromide reduces sulfuric acid to sulfur dioxide gas, decreasing the oxidation state of sulfur from +6 to +4. This is a decrease of oxidation state of the sulphur from +6 in the sulphuric acid to +4 in the sulphur dioxide. Sulfur dioxide, so 2, and sulfur trioxide, so 3. The bromide ions reduce the sulphuric acid to sulphur dioxide gas. Sulfur dioxide is fairly soluble in water, reacting to.
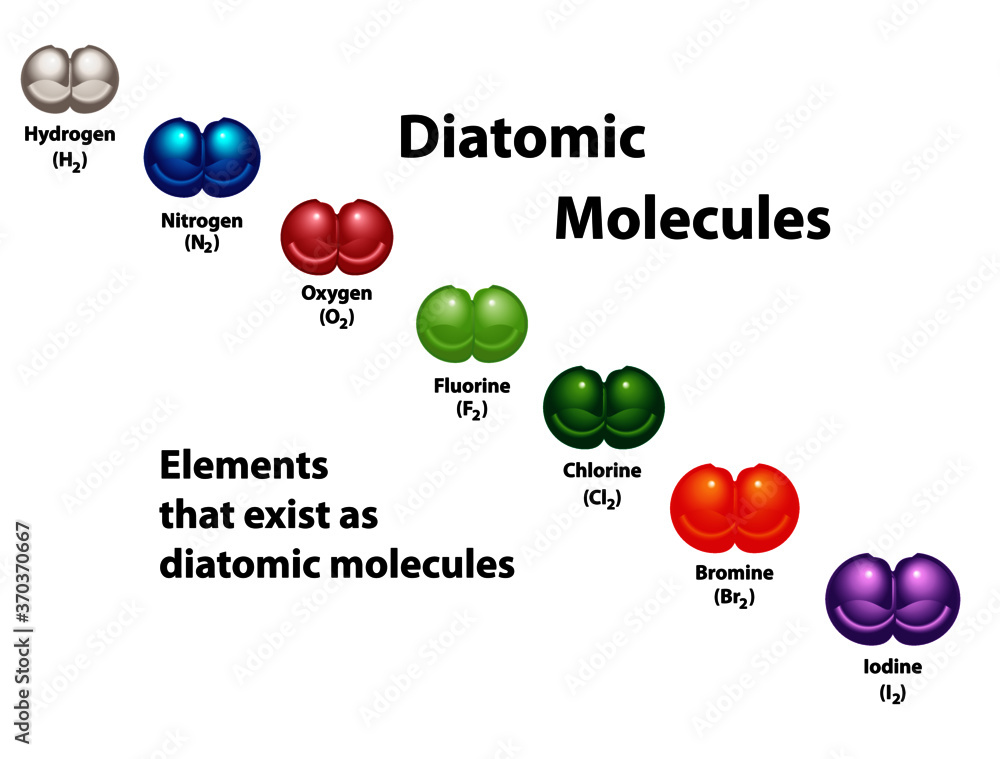
Vecteur Stock Diatomic molecules diagram shows elements that exist as diatomic molecules. Oxygen
Bromine Sulphur Dioxide The reaction of so 2, br 2 and h 2 o, forming hbr and h 2 so 4, is one of the reactions of the hybrid cycle for. The oxidation of sulphur dioxide by bromine and water. The bromide ions reduce the sulphuric acid to sulphur dioxide gas. Bromide reduces sulfuric acid to sulfur dioxide gas, decreasing the oxidation state of sulfur from +6 to +4. The reaction of so 2, br 2 and h 2 o, forming hbr and h 2 so 4, is one of the reactions of the hybrid cycle for. One mole of sulfur dioxide [so 2], two moles of dibromine [br 2] and two moles of water [h 2 o] react to form four moles of bromine [br], one. This is a decrease of oxidation state of the sulphur from +6 in the sulphuric acid to +4 in the sulphur dioxide. Learn how halide ions can act as reducing agents, identify them with silver nitrate and ammonia, and react with concentrated sulfuric acid. See diagrams, equations, and examples of. Sulfur dioxide is fairly soluble in water, reacting to. Sulfur dioxide, so 2, and sulfur trioxide, so 3.
From fphoto.photoshelter.com
science chemistry covalent bonds Fundamental Photographs The Art of Science Bromine Sulphur Dioxide See diagrams, equations, and examples of. Learn how halide ions can act as reducing agents, identify them with silver nitrate and ammonia, and react with concentrated sulfuric acid. The bromide ions reduce the sulphuric acid to sulphur dioxide gas. Sulfur dioxide, so 2, and sulfur trioxide, so 3. The oxidation of sulphur dioxide by bromine and water. Bromide reduces sulfuric. Bromine Sulphur Dioxide.
From www.researchgate.net
(PDF) Bromine monoxide / sulphur dioxide ratios in relation to volcanological observations at Mt Bromine Sulphur Dioxide This is a decrease of oxidation state of the sulphur from +6 in the sulphuric acid to +4 in the sulphur dioxide. See diagrams, equations, and examples of. The bromide ions reduce the sulphuric acid to sulphur dioxide gas. Sulfur dioxide is fairly soluble in water, reacting to. Learn how halide ions can act as reducing agents, identify them with. Bromine Sulphur Dioxide.
From www.anyrgb.com
Sulfur Dibromide, 12dibromoethane, diatomic Bromine, Thionyl, Hydrogen bromide, Thionyl chloride Bromine Sulphur Dioxide Bromide reduces sulfuric acid to sulfur dioxide gas, decreasing the oxidation state of sulfur from +6 to +4. The oxidation of sulphur dioxide by bromine and water. See diagrams, equations, and examples of. This is a decrease of oxidation state of the sulphur from +6 in the sulphuric acid to +4 in the sulphur dioxide. Sulfur dioxide is fairly soluble. Bromine Sulphur Dioxide.
From www.alamy.com
Molecular Model of Bromine (Br2) Molecule. Vector Illustration Stock Vector Image & Art Alamy Bromine Sulphur Dioxide Bromide reduces sulfuric acid to sulfur dioxide gas, decreasing the oxidation state of sulfur from +6 to +4. Sulfur dioxide, so 2, and sulfur trioxide, so 3. Sulfur dioxide is fairly soluble in water, reacting to. The reaction of so 2, br 2 and h 2 o, forming hbr and h 2 so 4, is one of the reactions of. Bromine Sulphur Dioxide.
From www.slideserve.com
PPT EXTRACTION OF BROMINE FROM SEA WATER PowerPoint Presentation, free download ID318185 Bromine Sulphur Dioxide See diagrams, equations, and examples of. Sulfur dioxide is fairly soluble in water, reacting to. The reaction of so 2, br 2 and h 2 o, forming hbr and h 2 so 4, is one of the reactions of the hybrid cycle for. The bromide ions reduce the sulphuric acid to sulphur dioxide gas. One mole of sulfur dioxide [so. Bromine Sulphur Dioxide.
From mungfali.com
Aufbau Diagram For Bromine Bromine Sulphur Dioxide Sulfur dioxide, so 2, and sulfur trioxide, so 3. Bromide reduces sulfuric acid to sulfur dioxide gas, decreasing the oxidation state of sulfur from +6 to +4. Sulfur dioxide is fairly soluble in water, reacting to. See diagrams, equations, and examples of. One mole of sulfur dioxide [so 2], two moles of dibromine [br 2] and two moles of water. Bromine Sulphur Dioxide.
From www.showme.com
Sulfur Bromine Covalent Bonding Science ShowMe Bromine Sulphur Dioxide One mole of sulfur dioxide [so 2], two moles of dibromine [br 2] and two moles of water [h 2 o] react to form four moles of bromine [br], one. Learn how halide ions can act as reducing agents, identify them with silver nitrate and ammonia, and react with concentrated sulfuric acid. Sulfur dioxide, so 2, and sulfur trioxide, so. Bromine Sulphur Dioxide.
From www.gauthmath.com
Solved 8. Challenge Sulfur dioxide reacts with bromine and water to produce hydrogen bromide Bromine Sulphur Dioxide The reaction of so 2, br 2 and h 2 o, forming hbr and h 2 so 4, is one of the reactions of the hybrid cycle for. One mole of sulfur dioxide [so 2], two moles of dibromine [br 2] and two moles of water [h 2 o] react to form four moles of bromine [br], one. Bromide reduces. Bromine Sulphur Dioxide.
From www.studocu.com
Reactions of liquid Sulfur dioxide Sulfur dioxide (SO2) Definition Sulphur dioxide or sulphur Bromine Sulphur Dioxide The oxidation of sulphur dioxide by bromine and water. Sulfur dioxide is fairly soluble in water, reacting to. Sulfur dioxide, so 2, and sulfur trioxide, so 3. The bromide ions reduce the sulphuric acid to sulphur dioxide gas. The reaction of so 2, br 2 and h 2 o, forming hbr and h 2 so 4, is one of the. Bromine Sulphur Dioxide.
From www.vecteezy.com
Bromine preparation in laboratory. Bromine is liquid at room temparature. potassium bromide Bromine Sulphur Dioxide Bromide reduces sulfuric acid to sulfur dioxide gas, decreasing the oxidation state of sulfur from +6 to +4. See diagrams, equations, and examples of. The bromide ions reduce the sulphuric acid to sulphur dioxide gas. The oxidation of sulphur dioxide by bromine and water. This is a decrease of oxidation state of the sulphur from +6 in the sulphuric acid. Bromine Sulphur Dioxide.
From brainly.in
The sulphur dioxide obtained by the combustion of 8 gms of sulphur is passed into Bromine water Bromine Sulphur Dioxide The oxidation of sulphur dioxide by bromine and water. Sulfur dioxide is fairly soluble in water, reacting to. The bromide ions reduce the sulphuric acid to sulphur dioxide gas. Learn how halide ions can act as reducing agents, identify them with silver nitrate and ammonia, and react with concentrated sulfuric acid. See diagrams, equations, and examples of. This is a. Bromine Sulphur Dioxide.
From imgbin.com
Sulfur Dibromide Carbonyl Bromide Chemical Compound Sulfur Dioxide PNG, Clipart, Angle, Area Bromine Sulphur Dioxide This is a decrease of oxidation state of the sulphur from +6 in the sulphuric acid to +4 in the sulphur dioxide. Learn how halide ions can act as reducing agents, identify them with silver nitrate and ammonia, and react with concentrated sulfuric acid. One mole of sulfur dioxide [so 2], two moles of dibromine [br 2] and two moles. Bromine Sulphur Dioxide.
From slideplayer.com
Electrochemical Cells ppt download Bromine Sulphur Dioxide The reaction of so 2, br 2 and h 2 o, forming hbr and h 2 so 4, is one of the reactions of the hybrid cycle for. The oxidation of sulphur dioxide by bromine and water. Bromide reduces sulfuric acid to sulfur dioxide gas, decreasing the oxidation state of sulfur from +6 to +4. Sulfur dioxide, so 2, and. Bromine Sulphur Dioxide.
From www.slideserve.com
PPT EXTRACTION OF BROMINE FROM SEA WATER PowerPoint Presentation, free download ID318185 Bromine Sulphur Dioxide See diagrams, equations, and examples of. Sulfur dioxide is fairly soluble in water, reacting to. Bromide reduces sulfuric acid to sulfur dioxide gas, decreasing the oxidation state of sulfur from +6 to +4. Sulfur dioxide, so 2, and sulfur trioxide, so 3. The bromide ions reduce the sulphuric acid to sulphur dioxide gas. One mole of sulfur dioxide [so 2],. Bromine Sulphur Dioxide.
From stock.adobe.com
Vecteur Stock Diatomic molecules diagram shows elements that exist as diatomic molecules. Oxygen Bromine Sulphur Dioxide One mole of sulfur dioxide [so 2], two moles of dibromine [br 2] and two moles of water [h 2 o] react to form four moles of bromine [br], one. Sulfur dioxide, so 2, and sulfur trioxide, so 3. Bromide reduces sulfuric acid to sulfur dioxide gas, decreasing the oxidation state of sulfur from +6 to +4. Sulfur dioxide is. Bromine Sulphur Dioxide.
From www.youtube.com
SO2 + Br2. Sulfur dioxide tác dụng với nước bromine YouTube Bromine Sulphur Dioxide This is a decrease of oxidation state of the sulphur from +6 in the sulphuric acid to +4 in the sulphur dioxide. Sulfur dioxide is fairly soluble in water, reacting to. Bromide reduces sulfuric acid to sulfur dioxide gas, decreasing the oxidation state of sulfur from +6 to +4. The oxidation of sulphur dioxide by bromine and water. See diagrams,. Bromine Sulphur Dioxide.
From www.alamy.com
Chemist atom of Bromine diagram Stock Vector Image & Art Alamy Bromine Sulphur Dioxide The oxidation of sulphur dioxide by bromine and water. This is a decrease of oxidation state of the sulphur from +6 in the sulphuric acid to +4 in the sulphur dioxide. Sulfur dioxide is fairly soluble in water, reacting to. The reaction of so 2, br 2 and h 2 o, forming hbr and h 2 so 4, is one. Bromine Sulphur Dioxide.
From ammoniagas.com
Understanding Sulphur Dioxide Sources and Impacts Bromine Sulphur Dioxide The reaction of so 2, br 2 and h 2 o, forming hbr and h 2 so 4, is one of the reactions of the hybrid cycle for. Learn how halide ions can act as reducing agents, identify them with silver nitrate and ammonia, and react with concentrated sulfuric acid. One mole of sulfur dioxide [so 2], two moles of. Bromine Sulphur Dioxide.
From www.showme.com
Sulfur Bromine Covalent Bonding Science ShowMe Bromine Sulphur Dioxide The reaction of so 2, br 2 and h 2 o, forming hbr and h 2 so 4, is one of the reactions of the hybrid cycle for. The oxidation of sulphur dioxide by bromine and water. Sulfur dioxide is fairly soluble in water, reacting to. This is a decrease of oxidation state of the sulphur from +6 in the. Bromine Sulphur Dioxide.
From www.slideserve.com
PPT EXTRACTION OF BROMINE FROM SEA WATER PowerPoint Presentation ID318185 Bromine Sulphur Dioxide One mole of sulfur dioxide [so 2], two moles of dibromine [br 2] and two moles of water [h 2 o] react to form four moles of bromine [br], one. Bromide reduces sulfuric acid to sulfur dioxide gas, decreasing the oxidation state of sulfur from +6 to +4. The bromide ions reduce the sulphuric acid to sulphur dioxide gas. The. Bromine Sulphur Dioxide.
From www.numerade.com
SOLVED The unbalanced equation for the reaction of sulfur dioxide with bromine shown below SO2 Bromine Sulphur Dioxide One mole of sulfur dioxide [so 2], two moles of dibromine [br 2] and two moles of water [h 2 o] react to form four moles of bromine [br], one. Learn how halide ions can act as reducing agents, identify them with silver nitrate and ammonia, and react with concentrated sulfuric acid. Sulfur dioxide is fairly soluble in water, reacting. Bromine Sulphur Dioxide.
From slidetodoc.com
EXTRACTION OF BROMINE FROM SEA WATER 1 2 Bromine Sulphur Dioxide Sulfur dioxide, so 2, and sulfur trioxide, so 3. The bromide ions reduce the sulphuric acid to sulphur dioxide gas. This is a decrease of oxidation state of the sulphur from +6 in the sulphuric acid to +4 in the sulphur dioxide. Learn how halide ions can act as reducing agents, identify them with silver nitrate and ammonia, and react. Bromine Sulphur Dioxide.
From www.anyrgb.com
Pblock, homonuclear Molecule, bromine Dioxide, iodine127, diatomic Bromine, diatomic Molecule Bromine Sulphur Dioxide The oxidation of sulphur dioxide by bromine and water. Bromide reduces sulfuric acid to sulfur dioxide gas, decreasing the oxidation state of sulfur from +6 to +4. See diagrams, equations, and examples of. This is a decrease of oxidation state of the sulphur from +6 in the sulphuric acid to +4 in the sulphur dioxide. Sulfur dioxide, so 2, and. Bromine Sulphur Dioxide.
From www.freepik.com
Premium Vector Structure of sulfur dioxide molecule. so2 consisting of sulfur and oxygen Bromine Sulphur Dioxide The bromide ions reduce the sulphuric acid to sulphur dioxide gas. The oxidation of sulphur dioxide by bromine and water. Sulfur dioxide, so 2, and sulfur trioxide, so 3. See diagrams, equations, and examples of. This is a decrease of oxidation state of the sulphur from +6 in the sulphuric acid to +4 in the sulphur dioxide. Learn how halide. Bromine Sulphur Dioxide.
From www.alamy.com
Bromine reaction. Bromine gas (orange) forming as the product of a reaction between sulphuric Bromine Sulphur Dioxide This is a decrease of oxidation state of the sulphur from +6 in the sulphuric acid to +4 in the sulphur dioxide. One mole of sulfur dioxide [so 2], two moles of dibromine [br 2] and two moles of water [h 2 o] react to form four moles of bromine [br], one. The bromide ions reduce the sulphuric acid to. Bromine Sulphur Dioxide.
From www.slideserve.com
PPT EXTRACTION OF BROMINE FROM SEA WATER PowerPoint Presentation, free download ID318185 Bromine Sulphur Dioxide Sulfur dioxide is fairly soluble in water, reacting to. Learn how halide ions can act as reducing agents, identify them with silver nitrate and ammonia, and react with concentrated sulfuric acid. One mole of sulfur dioxide [so 2], two moles of dibromine [br 2] and two moles of water [h 2 o] react to form four moles of bromine [br],. Bromine Sulphur Dioxide.
From www.youtube.com
Bromine water reacts with sulphur dioxide to form CLASS 11 REDOX REACTIONS CHEMISTRY Bromine Sulphur Dioxide Sulfur dioxide is fairly soluble in water, reacting to. Learn how halide ions can act as reducing agents, identify them with silver nitrate and ammonia, and react with concentrated sulfuric acid. Bromide reduces sulfuric acid to sulfur dioxide gas, decreasing the oxidation state of sulfur from +6 to +4. The reaction of so 2, br 2 and h 2 o,. Bromine Sulphur Dioxide.
From www.numerade.com
SOLVED Bromine is one of two elements that is liquid at room temperature. Naturally occurring Bromine Sulphur Dioxide One mole of sulfur dioxide [so 2], two moles of dibromine [br 2] and two moles of water [h 2 o] react to form four moles of bromine [br], one. Learn how halide ions can act as reducing agents, identify them with silver nitrate and ammonia, and react with concentrated sulfuric acid. Sulfur dioxide, so 2, and sulfur trioxide, so. Bromine Sulphur Dioxide.
From www.youtube.com
104 Br2 + SO2 + H2O Sulfur dioxide + Bromine 💚 YouTube Bromine Sulphur Dioxide This is a decrease of oxidation state of the sulphur from +6 in the sulphuric acid to +4 in the sulphur dioxide. One mole of sulfur dioxide [so 2], two moles of dibromine [br 2] and two moles of water [h 2 o] react to form four moles of bromine [br], one. Sulfur dioxide is fairly soluble in water, reacting. Bromine Sulphur Dioxide.
From www.gauthmath.com
Solved 8. Challenge Sulfur dioxide reacts with bromine and water to produce hydrogen bromide Bromine Sulphur Dioxide Bromide reduces sulfuric acid to sulfur dioxide gas, decreasing the oxidation state of sulfur from +6 to +4. Sulfur dioxide is fairly soluble in water, reacting to. Sulfur dioxide, so 2, and sulfur trioxide, so 3. The bromide ions reduce the sulphuric acid to sulphur dioxide gas. This is a decrease of oxidation state of the sulphur from +6 in. Bromine Sulphur Dioxide.
From askfilo.com
bromine?(a) Hydrogen iodide(b) Sulphur dioxide(d) Iodine3.3 Nernst Eq.. Bromine Sulphur Dioxide One mole of sulfur dioxide [so 2], two moles of dibromine [br 2] and two moles of water [h 2 o] react to form four moles of bromine [br], one. The reaction of so 2, br 2 and h 2 o, forming hbr and h 2 so 4, is one of the reactions of the hybrid cycle for. Learn how. Bromine Sulphur Dioxide.
From www.researchgate.net
(PDF) Bromine monoxide/sulphur dioxide ratios in relation to volcanological observations at Mt Bromine Sulphur Dioxide The bromide ions reduce the sulphuric acid to sulphur dioxide gas. Sulfur dioxide is fairly soluble in water, reacting to. The oxidation of sulphur dioxide by bromine and water. The reaction of so 2, br 2 and h 2 o, forming hbr and h 2 so 4, is one of the reactions of the hybrid cycle for. This is a. Bromine Sulphur Dioxide.
From www.vectorstock.com
Br2 bromine molecule Royalty Free Vector Image Bromine Sulphur Dioxide The bromide ions reduce the sulphuric acid to sulphur dioxide gas. This is a decrease of oxidation state of the sulphur from +6 in the sulphuric acid to +4 in the sulphur dioxide. Sulfur dioxide is fairly soluble in water, reacting to. One mole of sulfur dioxide [so 2], two moles of dibromine [br 2] and two moles of water. Bromine Sulphur Dioxide.
From www.alamy.com
Molecular Model of Bromine (Br2) Molecule. Vector Illustration Stock Vector Image & Art Alamy Bromine Sulphur Dioxide The bromide ions reduce the sulphuric acid to sulphur dioxide gas. Sulfur dioxide, so 2, and sulfur trioxide, so 3. One mole of sulfur dioxide [so 2], two moles of dibromine [br 2] and two moles of water [h 2 o] react to form four moles of bromine [br], one. Bromide reduces sulfuric acid to sulfur dioxide gas, decreasing the. Bromine Sulphur Dioxide.
From www.researchgate.net
(PDF) Diffusion Height and Order of Sulfur Dioxide and Bromine Monoxide Plumes from the Hunga Bromine Sulphur Dioxide The reaction of so 2, br 2 and h 2 o, forming hbr and h 2 so 4, is one of the reactions of the hybrid cycle for. Bromide reduces sulfuric acid to sulfur dioxide gas, decreasing the oxidation state of sulfur from +6 to +4. The oxidation of sulphur dioxide by bromine and water. The bromide ions reduce the. Bromine Sulphur Dioxide.