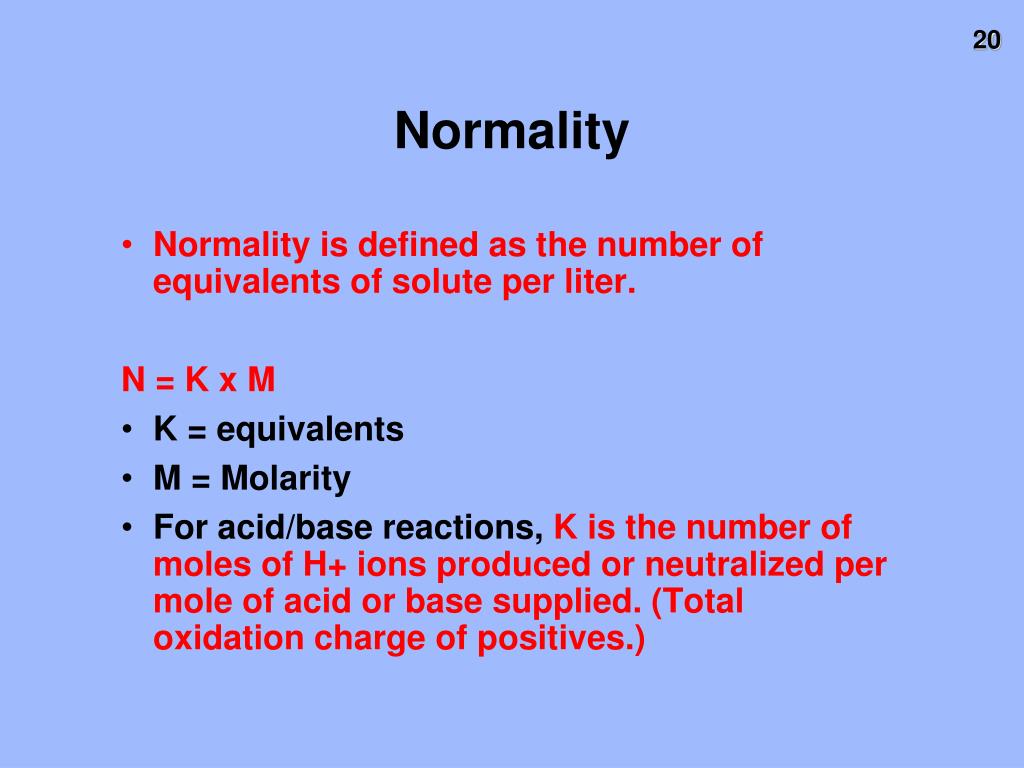
Normality Of Acid And Base . Normality is a measure of concentration based on the equivalents of reacting species. Learn how to calculate normality from molarity, equivalence factor. N 1 v 1 = n 2 v 2 where, n 1 = normality of the acidic solution v 1 = volume of the. Consider a 1 m solution of. Normality is a unit of concentration of a chemical solution based on the equivalent weight of a solute per liter. Normality is a measure of concentration based on the equivalents of reacting species. Learn how to calculate normality for different types of. Learn how to calculate normality from molarity and vice. Calculate how to prepare an acid or base solution of specified molarity or normality from a concentrated acid or base solution. Normality is a unit of concentration in chemistry that measures the number of gram equivalents of solute per litre of solution. To determine the normality of the acid and base titrations, use the following formula: Learn how to calculate normality, its relation with.
from www.slideserve.com
Learn how to calculate normality, its relation with. Learn how to calculate normality from molarity, equivalence factor. Calculate how to prepare an acid or base solution of specified molarity or normality from a concentrated acid or base solution. Normality is a measure of concentration based on the equivalents of reacting species. Normality is a measure of concentration based on the equivalents of reacting species. Learn how to calculate normality from molarity and vice. Learn how to calculate normality for different types of. Normality is a unit of concentration in chemistry that measures the number of gram equivalents of solute per litre of solution. To determine the normality of the acid and base titrations, use the following formula: Normality is a unit of concentration of a chemical solution based on the equivalent weight of a solute per liter.
PPT Lewis Bases, Acid Base Titration, and Normality PowerPoint Presentation ID6578470
Normality Of Acid And Base Calculate how to prepare an acid or base solution of specified molarity or normality from a concentrated acid or base solution. Normality is a unit of concentration in chemistry that measures the number of gram equivalents of solute per litre of solution. Learn how to calculate normality, its relation with. Normality is a measure of concentration based on the equivalents of reacting species. Consider a 1 m solution of. Normality is a unit of concentration of a chemical solution based on the equivalent weight of a solute per liter. Learn how to calculate normality for different types of. Learn how to calculate normality from molarity and vice. To determine the normality of the acid and base titrations, use the following formula: N 1 v 1 = n 2 v 2 where, n 1 = normality of the acidic solution v 1 = volume of the. Learn how to calculate normality from molarity, equivalence factor. Calculate how to prepare an acid or base solution of specified molarity or normality from a concentrated acid or base solution. Normality is a measure of concentration based on the equivalents of reacting species.
From mungfali.com
Acid Base Concept Map Normality Of Acid And Base Learn how to calculate normality for different types of. Normality is a unit of concentration in chemistry that measures the number of gram equivalents of solute per litre of solution. Normality is a unit of concentration of a chemical solution based on the equivalent weight of a solute per liter. To determine the normality of the acid and base titrations,. Normality Of Acid And Base.
From www.priyamstudycentre.com
Acids and Bases Definition, Concept, Theory, Examples Normality Of Acid And Base Learn how to calculate normality from molarity, equivalence factor. Normality is a unit of concentration in chemistry that measures the number of gram equivalents of solute per litre of solution. Consider a 1 m solution of. Normality is a measure of concentration based on the equivalents of reacting species. Calculate how to prepare an acid or base solution of specified. Normality Of Acid And Base.
From www.slideserve.com
PPT Lewis Bases, Acid Base Titration, and Normality PowerPoint Presentation ID6578470 Normality Of Acid And Base Consider a 1 m solution of. Normality is a measure of concentration based on the equivalents of reacting species. Learn how to calculate normality for different types of. Normality is a measure of concentration based on the equivalents of reacting species. Learn how to calculate normality, its relation with. Calculate how to prepare an acid or base solution of specified. Normality Of Acid And Base.
From www.thoughtco.com
How to Calculate Normality of a Solution Normality Of Acid And Base Normality is a unit of concentration of a chemical solution based on the equivalent weight of a solute per liter. Normality is a unit of concentration in chemistry that measures the number of gram equivalents of solute per litre of solution. To determine the normality of the acid and base titrations, use the following formula: Normality is a measure of. Normality Of Acid And Base.
From www.slideserve.com
PPT AcidBase calculations PowerPoint Presentation, free download ID2976295 Normality Of Acid And Base Learn how to calculate normality, its relation with. Normality is a measure of concentration based on the equivalents of reacting species. To determine the normality of the acid and base titrations, use the following formula: Normality is a measure of concentration based on the equivalents of reacting species. Learn how to calculate normality from molarity and vice. Learn how to. Normality Of Acid And Base.
From examples.yourdictionary.com
Difference Between Acids and Bases Key Properties Normality Of Acid And Base Calculate how to prepare an acid or base solution of specified molarity or normality from a concentrated acid or base solution. Learn how to calculate normality for different types of. Normality is a unit of concentration in chemistry that measures the number of gram equivalents of solute per litre of solution. Normality is a measure of concentration based on the. Normality Of Acid And Base.
From www.youtube.com
Normality of Acids and Bases in Chemistry YouTube Normality Of Acid And Base Normality is a unit of concentration of a chemical solution based on the equivalent weight of a solute per liter. Normality is a measure of concentration based on the equivalents of reacting species. Normality is a measure of concentration based on the equivalents of reacting species. Learn how to calculate normality from molarity and vice. Calculate how to prepare an. Normality Of Acid And Base.
From www.slideserve.com
PPT Lewis Bases, Acid Base Titration, and Normality PowerPoint Presentation ID6578470 Normality Of Acid And Base Learn how to calculate normality from molarity, equivalence factor. Normality is a unit of concentration of a chemical solution based on the equivalent weight of a solute per liter. Learn how to calculate normality for different types of. To determine the normality of the acid and base titrations, use the following formula: Learn how to calculate normality from molarity and. Normality Of Acid And Base.
From www.teachoo.com
Classification of Acids on Basis of source, Concentration Teachoo Normality Of Acid And Base Calculate how to prepare an acid or base solution of specified molarity or normality from a concentrated acid or base solution. N 1 v 1 = n 2 v 2 where, n 1 = normality of the acidic solution v 1 = volume of the. Normality is a measure of concentration based on the equivalents of reacting species. To determine. Normality Of Acid And Base.
From www.flinnsci.ca
AcidBase Strength Chart Normality Of Acid And Base To determine the normality of the acid and base titrations, use the following formula: Normality is a measure of concentration based on the equivalents of reacting species. Learn how to calculate normality from molarity and vice. Learn how to calculate normality from molarity, equivalence factor. Normality is a unit of concentration in chemistry that measures the number of gram equivalents. Normality Of Acid And Base.
From www.youtube.com
How To Calculate Normality & Equivalent Weight For Acid Base Reactions In Chemistry YouTube Normality Of Acid And Base Calculate how to prepare an acid or base solution of specified molarity or normality from a concentrated acid or base solution. Consider a 1 m solution of. Learn how to calculate normality for different types of. Normality is a measure of concentration based on the equivalents of reacting species. Normality is a unit of concentration of a chemical solution based. Normality Of Acid And Base.
From elife-news.blogspot.com
ELifes Calculation of Normality in acid base Normality Of Acid And Base N 1 v 1 = n 2 v 2 where, n 1 = normality of the acidic solution v 1 = volume of the. To determine the normality of the acid and base titrations, use the following formula: Learn how to calculate normality, its relation with. Normality is a measure of concentration based on the equivalents of reacting species. Normality. Normality Of Acid And Base.
From www.slideserve.com
PPT Acid Base Interpretation PowerPoint Presentation, free download ID4345930 Normality Of Acid And Base Normality is a unit of concentration of a chemical solution based on the equivalent weight of a solute per liter. N 1 v 1 = n 2 v 2 where, n 1 = normality of the acidic solution v 1 = volume of the. Learn how to calculate normality from molarity and vice. Consider a 1 m solution of. Calculate. Normality Of Acid And Base.
From www.slideserve.com
PPT Lewis Bases, Acid Base Titration, and Normality PowerPoint Presentation ID6578470 Normality Of Acid And Base Calculate how to prepare an acid or base solution of specified molarity or normality from a concentrated acid or base solution. N 1 v 1 = n 2 v 2 where, n 1 = normality of the acidic solution v 1 = volume of the. Learn how to calculate normality from molarity, equivalence factor. Learn how to calculate normality for. Normality Of Acid And Base.
From issr.edu.kh
pKa Values and strengths of Acids and Bases Normality Of Acid And Base Normality is a measure of concentration based on the equivalents of reacting species. Normality is a measure of concentration based on the equivalents of reacting species. Learn how to calculate normality for different types of. To determine the normality of the acid and base titrations, use the following formula: Normality is a unit of concentration in chemistry that measures the. Normality Of Acid And Base.
From sciencewithmrsb.weebly.com
Acids and Bases Science with Mrs Beggs Normality Of Acid And Base N 1 v 1 = n 2 v 2 where, n 1 = normality of the acidic solution v 1 = volume of the. Learn how to calculate normality, its relation with. Calculate how to prepare an acid or base solution of specified molarity or normality from a concentrated acid or base solution. Normality is a unit of concentration of. Normality Of Acid And Base.
From www.slideserve.com
PPT Acid Base Balance PowerPoint Presentation, free download ID3199429 Normality Of Acid And Base Normality is a unit of concentration in chemistry that measures the number of gram equivalents of solute per litre of solution. Learn how to calculate normality from molarity and vice. Calculate how to prepare an acid or base solution of specified molarity or normality from a concentrated acid or base solution. Normality is a unit of concentration of a chemical. Normality Of Acid And Base.
From courses.lumenlearning.com
Relative Strengths of Acids and Bases Chemistry Normality Of Acid And Base N 1 v 1 = n 2 v 2 where, n 1 = normality of the acidic solution v 1 = volume of the. Learn how to calculate normality from molarity, equivalence factor. Learn how to calculate normality, its relation with. Normality is a measure of concentration based on the equivalents of reacting species. Calculate how to prepare an acid. Normality Of Acid And Base.
From www.slideserve.com
PPT Lewis Bases, Acid Base Titration, and Normality PowerPoint Presentation ID6578470 Normality Of Acid And Base Learn how to calculate normality for different types of. To determine the normality of the acid and base titrations, use the following formula: Normality is a unit of concentration of a chemical solution based on the equivalent weight of a solute per liter. N 1 v 1 = n 2 v 2 where, n 1 = normality of the acidic. Normality Of Acid And Base.
From www.slideserve.com
PPT Unit 19 Acid Base Equilibria Titrations PowerPoint Presentation ID4401864 Normality Of Acid And Base Learn how to calculate normality from molarity and vice. Calculate how to prepare an acid or base solution of specified molarity or normality from a concentrated acid or base solution. To determine the normality of the acid and base titrations, use the following formula: Normality is a measure of concentration based on the equivalents of reacting species. Normality is a. Normality Of Acid And Base.
From stock.adobe.com
Stockvector Acids Bases and Salts infographic diagram showing solution with comparison table and Normality Of Acid And Base Normality is a unit of concentration in chemistry that measures the number of gram equivalents of solute per litre of solution. Normality is a measure of concentration based on the equivalents of reacting species. Calculate how to prepare an acid or base solution of specified molarity or normality from a concentrated acid or base solution. Normality is a measure of. Normality Of Acid And Base.
From www.slideserve.com
PPT Lewis Bases, Acid Base Titration, and Normality PowerPoint Presentation ID6578470 Normality Of Acid And Base Consider a 1 m solution of. Learn how to calculate normality from molarity and vice. Normality is a unit of concentration in chemistry that measures the number of gram equivalents of solute per litre of solution. N 1 v 1 = n 2 v 2 where, n 1 = normality of the acidic solution v 1 = volume of the.. Normality Of Acid And Base.
From www.worksheetsplanet.com
Acid and Bases Differences Normality Of Acid And Base Learn how to calculate normality, its relation with. Normality is a unit of concentration in chemistry that measures the number of gram equivalents of solute per litre of solution. Learn how to calculate normality from molarity and vice. Calculate how to prepare an acid or base solution of specified molarity or normality from a concentrated acid or base solution. Learn. Normality Of Acid And Base.
From www.youtube.com
(Analytical Chemistry2)pH of Mixture of acids & bases, Relation between Molality,Molarity Normality Of Acid And Base Normality is a unit of concentration in chemistry that measures the number of gram equivalents of solute per litre of solution. Normality is a measure of concentration based on the equivalents of reacting species. Learn how to calculate normality from molarity and vice. Learn how to calculate normality from molarity, equivalence factor. Learn how to calculate normality for different types. Normality Of Acid And Base.
From www.slideserve.com
PPT Chapter 16 Acids and Bases PowerPoint Presentation, free download ID5408786 Normality Of Acid And Base To determine the normality of the acid and base titrations, use the following formula: Learn how to calculate normality, its relation with. Normality is a unit of concentration of a chemical solution based on the equivalent weight of a solute per liter. Learn how to calculate normality for different types of. Normality is a measure of concentration based on the. Normality Of Acid And Base.
From sciencenotes.org
Facts About Acids and Bases Normality Of Acid And Base Learn how to calculate normality from molarity, equivalence factor. N 1 v 1 = n 2 v 2 where, n 1 = normality of the acidic solution v 1 = volume of the. Normality is a measure of concentration based on the equivalents of reacting species. To determine the normality of the acid and base titrations, use the following formula:. Normality Of Acid And Base.
From www.sigmaaldrich.com
Acid and Base Chart — Table of Acids & Bases Normality Of Acid And Base To determine the normality of the acid and base titrations, use the following formula: Learn how to calculate normality from molarity, equivalence factor. Calculate how to prepare an acid or base solution of specified molarity or normality from a concentrated acid or base solution. Learn how to calculate normality for different types of. Consider a 1 m solution of. N. Normality Of Acid And Base.
From www.slideserve.com
PPT ACIDS AND BASES PowerPoint Presentation, free download ID2145347 Normality Of Acid And Base Learn how to calculate normality from molarity and vice. To determine the normality of the acid and base titrations, use the following formula: Normality is a unit of concentration of a chemical solution based on the equivalent weight of a solute per liter. Calculate how to prepare an acid or base solution of specified molarity or normality from a concentrated. Normality Of Acid And Base.
From www.slideserve.com
PPT Acid, Base, Electrolytes PowerPoint Presentation, free download ID3058417 Normality Of Acid And Base Normality is a measure of concentration based on the equivalents of reacting species. Calculate how to prepare an acid or base solution of specified molarity or normality from a concentrated acid or base solution. To determine the normality of the acid and base titrations, use the following formula: Consider a 1 m solution of. Learn how to calculate normality, its. Normality Of Acid And Base.
From www.youtube.com
Normality(1) Acid Base Titration YouTube Normality Of Acid And Base Normality is a measure of concentration based on the equivalents of reacting species. N 1 v 1 = n 2 v 2 where, n 1 = normality of the acidic solution v 1 = volume of the. Consider a 1 m solution of. Normality is a unit of concentration in chemistry that measures the number of gram equivalents of solute. Normality Of Acid And Base.
From byjus.com
If the Normality of acid and base is equal then what is the Normality of mixture? Normality Of Acid And Base To determine the normality of the acid and base titrations, use the following formula: Learn how to calculate normality for different types of. Normality is a measure of concentration based on the equivalents of reacting species. Consider a 1 m solution of. Normality is a unit of concentration in chemistry that measures the number of gram equivalents of solute per. Normality Of Acid And Base.
From www.pinterest.com
How to Calculate Normality of a Solution Chemistry lessons, Chemistry, Solutions Normality Of Acid And Base Normality is a unit of concentration of a chemical solution based on the equivalent weight of a solute per liter. Learn how to calculate normality for different types of. To determine the normality of the acid and base titrations, use the following formula: Learn how to calculate normality from molarity and vice. Normality is a measure of concentration based on. Normality Of Acid And Base.
From straighthealthcare.com
Normal acidbase physiology illustration Normality Of Acid And Base Normality is a measure of concentration based on the equivalents of reacting species. Learn how to calculate normality, its relation with. Normality is a unit of concentration of a chemical solution based on the equivalent weight of a solute per liter. Calculate how to prepare an acid or base solution of specified molarity or normality from a concentrated acid or. Normality Of Acid And Base.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID3842068 Normality Of Acid And Base N 1 v 1 = n 2 v 2 where, n 1 = normality of the acidic solution v 1 = volume of the. Consider a 1 m solution of. Learn how to calculate normality from molarity and vice. Normality is a unit of concentration in chemistry that measures the number of gram equivalents of solute per litre of solution.. Normality Of Acid And Base.
From www.slideserve.com
PPT Lewis Bases, Acid Base Titration, and Normality PowerPoint Presentation ID6578470 Normality Of Acid And Base N 1 v 1 = n 2 v 2 where, n 1 = normality of the acidic solution v 1 = volume of the. To determine the normality of the acid and base titrations, use the following formula: Consider a 1 m solution of. Learn how to calculate normality from molarity and vice. Learn how to calculate normality from molarity,. Normality Of Acid And Base.