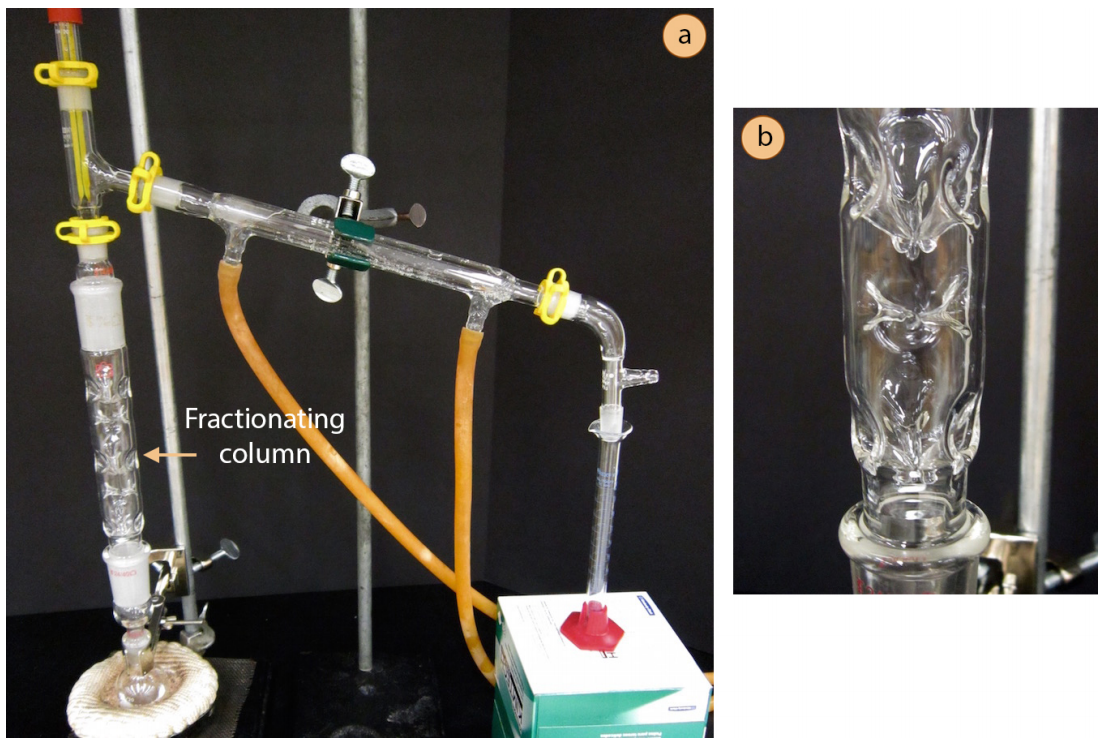
Boiling Chips In Fractional Distillation . Each fraction consists of groups of hydrocarbons of similar chain lengths. Pour the distillate collected to round bottom flask. They will prevent superheating of the liquid being distilled and they will cause a more controlled boil,. The concepts of a fractional distillation can be shown through a distillation curve. The mixture is separated into fractions, based on boiling points; Fractional distillation is used in oil refineries. Fractional distillation is used for both oil refining and purification of reagents and products. Set up the fractional distillation. To promote even heating of the liquid, a boiling chip or a magnetic stir bar is added before heat is applied to the distillation flask. When the difference in boiling. Collect the distillate every 0.5 ml. Boiling chips should be placed in the distillation flask for two reasons: A simple distillation is incapable of significant purification if the boiling points of the components are too close.
from chem.libretexts.org
Pour the distillate collected to round bottom flask. Fractional distillation is used in oil refineries. To promote even heating of the liquid, a boiling chip or a magnetic stir bar is added before heat is applied to the distillation flask. Fractional distillation is used for both oil refining and purification of reagents and products. When the difference in boiling. Collect the distillate every 0.5 ml. Set up the fractional distillation. The concepts of a fractional distillation can be shown through a distillation curve. Boiling chips should be placed in the distillation flask for two reasons: A simple distillation is incapable of significant purification if the boiling points of the components are too close.
5.3A Theory of Fractional Distillation Chemistry LibreTexts
Boiling Chips In Fractional Distillation Each fraction consists of groups of hydrocarbons of similar chain lengths. The concepts of a fractional distillation can be shown through a distillation curve. Collect the distillate every 0.5 ml. A simple distillation is incapable of significant purification if the boiling points of the components are too close. They will prevent superheating of the liquid being distilled and they will cause a more controlled boil,. To promote even heating of the liquid, a boiling chip or a magnetic stir bar is added before heat is applied to the distillation flask. Boiling chips should be placed in the distillation flask for two reasons: Fractional distillation is used for both oil refining and purification of reagents and products. Set up the fractional distillation. Each fraction consists of groups of hydrocarbons of similar chain lengths. Pour the distillate collected to round bottom flask. When the difference in boiling. The mixture is separated into fractions, based on boiling points; Fractional distillation is used in oil refineries.
From testbook.com
Fractional Distillation Learn Definition, Principle, Procedure Boiling Chips In Fractional Distillation Each fraction consists of groups of hydrocarbons of similar chain lengths. Set up the fractional distillation. The mixture is separated into fractions, based on boiling points; Boiling chips should be placed in the distillation flask for two reasons: Collect the distillate every 0.5 ml. Fractional distillation is used in oil refineries. Pour the distillate collected to round bottom flask. They. Boiling Chips In Fractional Distillation.
From mungfali.com
Fractional Distillation Labelled Diagram Boiling Chips In Fractional Distillation Fractional distillation is used in oil refineries. A simple distillation is incapable of significant purification if the boiling points of the components are too close. They will prevent superheating of the liquid being distilled and they will cause a more controlled boil,. Boiling chips should be placed in the distillation flask for two reasons: Each fraction consists of groups of. Boiling Chips In Fractional Distillation.
From chembam.com
Purification by fractional distillation Boiling Chips In Fractional Distillation Boiling chips should be placed in the distillation flask for two reasons: Collect the distillate every 0.5 ml. The concepts of a fractional distillation can be shown through a distillation curve. They will prevent superheating of the liquid being distilled and they will cause a more controlled boil,. Fractional distillation is used for both oil refining and purification of reagents. Boiling Chips In Fractional Distillation.
From www.chemicals.co.uk
What is Fractional Distillation? The Chemistry Blog Boiling Chips In Fractional Distillation Pour the distillate collected to round bottom flask. The mixture is separated into fractions, based on boiling points; To promote even heating of the liquid, a boiling chip or a magnetic stir bar is added before heat is applied to the distillation flask. Fractional distillation is used in oil refineries. They will prevent superheating of the liquid being distilled and. Boiling Chips In Fractional Distillation.
From www.savemyexams.com
Fractional Distillation & Petrochemicals AQA GCSE Chemistry Combined Boiling Chips In Fractional Distillation A simple distillation is incapable of significant purification if the boiling points of the components are too close. Each fraction consists of groups of hydrocarbons of similar chain lengths. Set up the fractional distillation. They will prevent superheating of the liquid being distilled and they will cause a more controlled boil,. The concepts of a fractional distillation can be shown. Boiling Chips In Fractional Distillation.
From ar.inspiredpencil.com
Fractional Distillation Diagram Labeled Boiling Chips In Fractional Distillation The mixture is separated into fractions, based on boiling points; Boiling chips should be placed in the distillation flask for two reasons: Fractional distillation is used for both oil refining and purification of reagents and products. Set up the fractional distillation. When the difference in boiling. Each fraction consists of groups of hydrocarbons of similar chain lengths. Collect the distillate. Boiling Chips In Fractional Distillation.
From www.slideserve.com
PPT Fractional Distillation PowerPoint Presentation ID6021367 Boiling Chips In Fractional Distillation The concepts of a fractional distillation can be shown through a distillation curve. Fractional distillation is used in oil refineries. They will prevent superheating of the liquid being distilled and they will cause a more controlled boil,. Pour the distillate collected to round bottom flask. Each fraction consists of groups of hydrocarbons of similar chain lengths. To promote even heating. Boiling Chips In Fractional Distillation.
From www.vedantu.com
Uses of Distillation Learn Important Terms and Concepts Boiling Chips In Fractional Distillation To promote even heating of the liquid, a boiling chip or a magnetic stir bar is added before heat is applied to the distillation flask. Set up the fractional distillation. Pour the distillate collected to round bottom flask. Fractional distillation is used for both oil refining and purification of reagents and products. Each fraction consists of groups of hydrocarbons of. Boiling Chips In Fractional Distillation.
From www.jove.com
Fractional Distillation Principle, Purification of a Mixture Organic Boiling Chips In Fractional Distillation Each fraction consists of groups of hydrocarbons of similar chain lengths. When the difference in boiling. Fractional distillation is used in oil refineries. Pour the distillate collected to round bottom flask. Fractional distillation is used for both oil refining and purification of reagents and products. To promote even heating of the liquid, a boiling chip or a magnetic stir bar. Boiling Chips In Fractional Distillation.
From mungfali.com
Fractional Distillation Labelled Diagram Boiling Chips In Fractional Distillation The concepts of a fractional distillation can be shown through a distillation curve. Pour the distillate collected to round bottom flask. When the difference in boiling. Fractional distillation is used for both oil refining and purification of reagents and products. To promote even heating of the liquid, a boiling chip or a magnetic stir bar is added before heat is. Boiling Chips In Fractional Distillation.
From www.slideserve.com
PPT Fractional Distillation PowerPoint Presentation, free download Boiling Chips In Fractional Distillation A simple distillation is incapable of significant purification if the boiling points of the components are too close. Fractional distillation is used for both oil refining and purification of reagents and products. Set up the fractional distillation. When the difference in boiling. The mixture is separated into fractions, based on boiling points; The concepts of a fractional distillation can be. Boiling Chips In Fractional Distillation.
From www.dreamstime.com
Fractional Distillation is a Process Used To Separate a Mixture of Two Boiling Chips In Fractional Distillation They will prevent superheating of the liquid being distilled and they will cause a more controlled boil,. Collect the distillate every 0.5 ml. Fractional distillation is used in oil refineries. Pour the distillate collected to round bottom flask. Each fraction consists of groups of hydrocarbons of similar chain lengths. Fractional distillation is used for both oil refining and purification of. Boiling Chips In Fractional Distillation.
From mavink.com
Labelled Diagram Of Distillation Boiling Chips In Fractional Distillation To promote even heating of the liquid, a boiling chip or a magnetic stir bar is added before heat is applied to the distillation flask. When the difference in boiling. Pour the distillate collected to round bottom flask. Collect the distillate every 0.5 ml. A simple distillation is incapable of significant purification if the boiling points of the components are. Boiling Chips In Fractional Distillation.
From studiousguy.com
6 Fractional Distillation Examples in Everyday Life StudiousGuy Boiling Chips In Fractional Distillation Fractional distillation is used for both oil refining and purification of reagents and products. Set up the fractional distillation. Collect the distillate every 0.5 ml. When the difference in boiling. The concepts of a fractional distillation can be shown through a distillation curve. A simple distillation is incapable of significant purification if the boiling points of the components are too. Boiling Chips In Fractional Distillation.
From www.chemicalslearning.com
Fractional Distillation Work for the Crude Oil Boiling Chips In Fractional Distillation Pour the distillate collected to round bottom flask. The mixture is separated into fractions, based on boiling points; Set up the fractional distillation. Each fraction consists of groups of hydrocarbons of similar chain lengths. To promote even heating of the liquid, a boiling chip or a magnetic stir bar is added before heat is applied to the distillation flask. The. Boiling Chips In Fractional Distillation.
From chemicalengineeringworld.com
Fractional Distillation Process Chemical Engineering World Boiling Chips In Fractional Distillation Pour the distillate collected to round bottom flask. They will prevent superheating of the liquid being distilled and they will cause a more controlled boil,. Fractional distillation is used in oil refineries. The mixture is separated into fractions, based on boiling points; When the difference in boiling. A simple distillation is incapable of significant purification if the boiling points of. Boiling Chips In Fractional Distillation.
From studylib.net
Fractional Distillation Boiling Chips In Fractional Distillation A simple distillation is incapable of significant purification if the boiling points of the components are too close. Collect the distillate every 0.5 ml. To promote even heating of the liquid, a boiling chip or a magnetic stir bar is added before heat is applied to the distillation flask. Set up the fractional distillation. They will prevent superheating of the. Boiling Chips In Fractional Distillation.
From mavink.com
Fractional Distillation Labeled Diagram Boiling Chips In Fractional Distillation Boiling chips should be placed in the distillation flask for two reasons: Fractional distillation is used for both oil refining and purification of reagents and products. Set up the fractional distillation. Collect the distillate every 0.5 ml. To promote even heating of the liquid, a boiling chip or a magnetic stir bar is added before heat is applied to the. Boiling Chips In Fractional Distillation.
From slideplayer.com
Separation Techniques in Chemistry ppt download Boiling Chips In Fractional Distillation Each fraction consists of groups of hydrocarbons of similar chain lengths. To promote even heating of the liquid, a boiling chip or a magnetic stir bar is added before heat is applied to the distillation flask. When the difference in boiling. Fractional distillation is used in oil refineries. Set up the fractional distillation. Collect the distillate every 0.5 ml. The. Boiling Chips In Fractional Distillation.
From pixels.com
Fractional Distillation Apparatus Photograph by Martyn F. Chillmaid Boiling Chips In Fractional Distillation The concepts of a fractional distillation can be shown through a distillation curve. They will prevent superheating of the liquid being distilled and they will cause a more controlled boil,. A simple distillation is incapable of significant purification if the boiling points of the components are too close. Each fraction consists of groups of hydrocarbons of similar chain lengths. Fractional. Boiling Chips In Fractional Distillation.
From www.slideserve.com
PPT Boiling Points Distillations PowerPoint Presentation, free Boiling Chips In Fractional Distillation Pour the distillate collected to round bottom flask. When the difference in boiling. The mixture is separated into fractions, based on boiling points; Set up the fractional distillation. Boiling chips should be placed in the distillation flask for two reasons: To promote even heating of the liquid, a boiling chip or a magnetic stir bar is added before heat is. Boiling Chips In Fractional Distillation.
From chem.libretexts.org
5.3A Theory of Fractional Distillation Chemistry LibreTexts Boiling Chips In Fractional Distillation Boiling chips should be placed in the distillation flask for two reasons: A simple distillation is incapable of significant purification if the boiling points of the components are too close. Fractional distillation is used for both oil refining and purification of reagents and products. When the difference in boiling. Pour the distillate collected to round bottom flask. The concepts of. Boiling Chips In Fractional Distillation.
From chemnotcheem.com
Simple distillation vs fractional distillation O Level Chemistry Notes Boiling Chips In Fractional Distillation Collect the distillate every 0.5 ml. A simple distillation is incapable of significant purification if the boiling points of the components are too close. When the difference in boiling. Fractional distillation is used for both oil refining and purification of reagents and products. Fractional distillation is used in oil refineries. Boiling chips should be placed in the distillation flask for. Boiling Chips In Fractional Distillation.
From www.chemicals.co.uk
What is Fractional Distillation? The Chemistry Blog Boiling Chips In Fractional Distillation Set up the fractional distillation. They will prevent superheating of the liquid being distilled and they will cause a more controlled boil,. Boiling chips should be placed in the distillation flask for two reasons: A simple distillation is incapable of significant purification if the boiling points of the components are too close. Fractional distillation is used for both oil refining. Boiling Chips In Fractional Distillation.
From psiberg.com
Fractional Distillation Uses, Working, and Apparatus PSIBERG Boiling Chips In Fractional Distillation Pour the distillate collected to round bottom flask. Collect the distillate every 0.5 ml. Fractional distillation is used in oil refineries. A simple distillation is incapable of significant purification if the boiling points of the components are too close. They will prevent superheating of the liquid being distilled and they will cause a more controlled boil,. Each fraction consists of. Boiling Chips In Fractional Distillation.
From psiberg.com
Fractional Distillation Uses, Working, and Apparatus PSIBERG Boiling Chips In Fractional Distillation Set up the fractional distillation. They will prevent superheating of the liquid being distilled and they will cause a more controlled boil,. Each fraction consists of groups of hydrocarbons of similar chain lengths. To promote even heating of the liquid, a boiling chip or a magnetic stir bar is added before heat is applied to the distillation flask. Fractional distillation. Boiling Chips In Fractional Distillation.
From www.gustawater.com
The Ultimate Guide to Fractional Distillation Boiling Chips In Fractional Distillation Each fraction consists of groups of hydrocarbons of similar chain lengths. Pour the distillate collected to round bottom flask. They will prevent superheating of the liquid being distilled and they will cause a more controlled boil,. Set up the fractional distillation. Fractional distillation is used for both oil refining and purification of reagents and products. Fractional distillation is used in. Boiling Chips In Fractional Distillation.
From www.sciencephoto.com
Boiling Chip Stock Image C011/9135 Science Photo Library Boiling Chips In Fractional Distillation Boiling chips should be placed in the distillation flask for two reasons: They will prevent superheating of the liquid being distilled and they will cause a more controlled boil,. The concepts of a fractional distillation can be shown through a distillation curve. Each fraction consists of groups of hydrocarbons of similar chain lengths. The mixture is separated into fractions, based. Boiling Chips In Fractional Distillation.
From www.elevise.co.uk
C1 I) Simple & Fractional Distillation AQA Combined Science Trilogy Boiling Chips In Fractional Distillation Set up the fractional distillation. Pour the distillate collected to round bottom flask. The concepts of a fractional distillation can be shown through a distillation curve. Each fraction consists of groups of hydrocarbons of similar chain lengths. The mixture is separated into fractions, based on boiling points; They will prevent superheating of the liquid being distilled and they will cause. Boiling Chips In Fractional Distillation.
From www.slideshare.net
Fractional distillation Boiling Chips In Fractional Distillation They will prevent superheating of the liquid being distilled and they will cause a more controlled boil,. Each fraction consists of groups of hydrocarbons of similar chain lengths. The mixture is separated into fractions, based on boiling points; Collect the distillate every 0.5 ml. Fractional distillation is used for both oil refining and purification of reagents and products. Fractional distillation. Boiling Chips In Fractional Distillation.
From keystagewiki.com
Fractional Distillation Key Stage Wiki Boiling Chips In Fractional Distillation Fractional distillation is used in oil refineries. The mixture is separated into fractions, based on boiling points; To promote even heating of the liquid, a boiling chip or a magnetic stir bar is added before heat is applied to the distillation flask. They will prevent superheating of the liquid being distilled and they will cause a more controlled boil,. When. Boiling Chips In Fractional Distillation.
From www.nagwa.com
Question Video Identifying a Piece of Apparatus Used in Fractional Boiling Chips In Fractional Distillation Each fraction consists of groups of hydrocarbons of similar chain lengths. Pour the distillate collected to round bottom flask. They will prevent superheating of the liquid being distilled and they will cause a more controlled boil,. The concepts of a fractional distillation can be shown through a distillation curve. To promote even heating of the liquid, a boiling chip or. Boiling Chips In Fractional Distillation.
From www.solnpharma.com
Fractional Distillation How it Works? Boiling Chips In Fractional Distillation When the difference in boiling. Each fraction consists of groups of hydrocarbons of similar chain lengths. To promote even heating of the liquid, a boiling chip or a magnetic stir bar is added before heat is applied to the distillation flask. Fractional distillation is used for both oil refining and purification of reagents and products. The mixture is separated into. Boiling Chips In Fractional Distillation.
From www.youtube.com
Fractional distillation diagram / science diagram / important science Boiling Chips In Fractional Distillation Each fraction consists of groups of hydrocarbons of similar chain lengths. Boiling chips should be placed in the distillation flask for two reasons: Collect the distillate every 0.5 ml. Fractional distillation is used for both oil refining and purification of reagents and products. The mixture is separated into fractions, based on boiling points; Set up the fractional distillation. Fractional distillation. Boiling Chips In Fractional Distillation.
From www.elevise.co.uk
C1 I) Simple & Fractional Distillation AQA Combined Science Trilogy Boiling Chips In Fractional Distillation A simple distillation is incapable of significant purification if the boiling points of the components are too close. The concepts of a fractional distillation can be shown through a distillation curve. To promote even heating of the liquid, a boiling chip or a magnetic stir bar is added before heat is applied to the distillation flask. Fractional distillation is used. Boiling Chips In Fractional Distillation.