Medical Device Regulatory Strategy Example . Learn how your company can form a regulatory strategy for your medical device in 8 easy steps. Read our tips for preparing a regulatory strategy for medical devices in the united states (us) and the european union (eu). Here’s what to consider when planning one. Your medical device global regulatory strategy can have a huge impact on the future success of your product. We provide a review of emerging strategies, opportunities, and best practices to increase the regulatory knowledge base and. Raps has released the second edition of its global medical device regulatory strategy book, providing a @how to@ guide to. It involves carefully selecting the best markets and pathways for both your device and your business. It goes beyond simply choosing a pathway to market;
from apacmed.org
Learn how your company can form a regulatory strategy for your medical device in 8 easy steps. Read our tips for preparing a regulatory strategy for medical devices in the united states (us) and the european union (eu). It involves carefully selecting the best markets and pathways for both your device and your business. We provide a review of emerging strategies, opportunities, and best practices to increase the regulatory knowledge base and. Raps has released the second edition of its global medical device regulatory strategy book, providing a @how to@ guide to. It goes beyond simply choosing a pathway to market; Your medical device global regulatory strategy can have a huge impact on the future success of your product. Here’s what to consider when planning one.
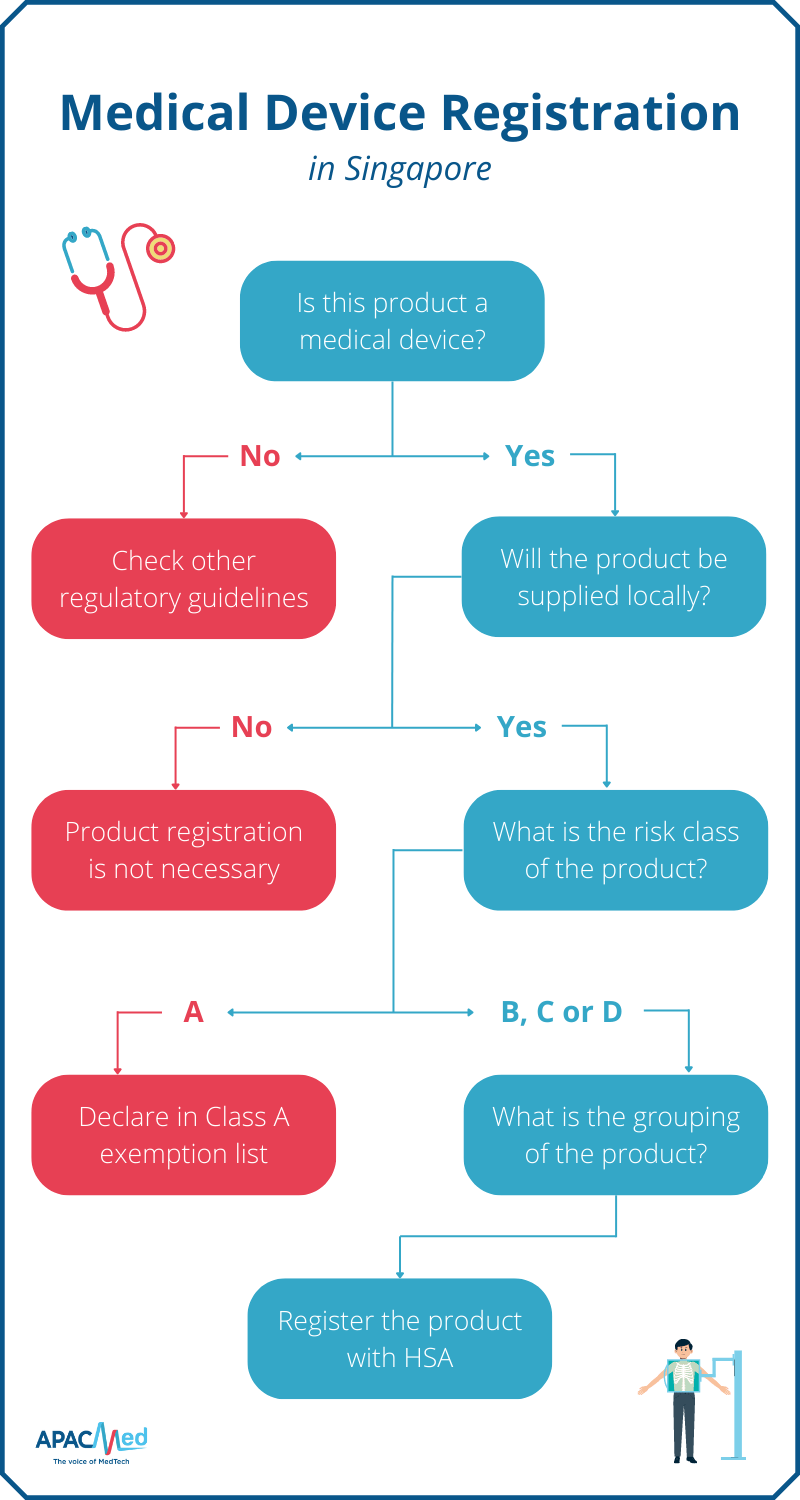
Medical Device Regulation Importance and Examples in APAC
Medical Device Regulatory Strategy Example We provide a review of emerging strategies, opportunities, and best practices to increase the regulatory knowledge base and. Raps has released the second edition of its global medical device regulatory strategy book, providing a @how to@ guide to. Your medical device global regulatory strategy can have a huge impact on the future success of your product. Read our tips for preparing a regulatory strategy for medical devices in the united states (us) and the european union (eu). Here’s what to consider when planning one. Learn how your company can form a regulatory strategy for your medical device in 8 easy steps. We provide a review of emerging strategies, opportunities, and best practices to increase the regulatory knowledge base and. It involves carefully selecting the best markets and pathways for both your device and your business. It goes beyond simply choosing a pathway to market;
From www.meddeviceonline.com
World Health Organization (WHO) Unveils Global Regulatory Framework For Medical Device Regulatory Strategy Example Read our tips for preparing a regulatory strategy for medical devices in the united states (us) and the european union (eu). Learn how your company can form a regulatory strategy for your medical device in 8 easy steps. It involves carefully selecting the best markets and pathways for both your device and your business. Your medical device global regulatory strategy. Medical Device Regulatory Strategy Example.
From www.cascade.app
Regulatory Strategy Template Medical Device Regulatory Strategy Example Your medical device global regulatory strategy can have a huge impact on the future success of your product. We provide a review of emerging strategies, opportunities, and best practices to increase the regulatory knowledge base and. Here’s what to consider when planning one. Raps has released the second edition of its global medical device regulatory strategy book, providing a @how. Medical Device Regulatory Strategy Example.
From apacmed.org
Medical Device Regulation Importance and Examples in APAC Medical Device Regulatory Strategy Example Read our tips for preparing a regulatory strategy for medical devices in the united states (us) and the european union (eu). Here’s what to consider when planning one. It involves carefully selecting the best markets and pathways for both your device and your business. We provide a review of emerging strategies, opportunities, and best practices to increase the regulatory knowledge. Medical Device Regulatory Strategy Example.
From www.sycaimedical.com
Overview on the regulatory path for software medical devices Medical Device Regulatory Strategy Example It involves carefully selecting the best markets and pathways for both your device and your business. Here’s what to consider when planning one. Read our tips for preparing a regulatory strategy for medical devices in the united states (us) and the european union (eu). We provide a review of emerging strategies, opportunities, and best practices to increase the regulatory knowledge. Medical Device Regulatory Strategy Example.
From operonstrategist.com
Regulatory strategist Know Everything Before Introduce any Device Medical Device Regulatory Strategy Example We provide a review of emerging strategies, opportunities, and best practices to increase the regulatory knowledge base and. It goes beyond simply choosing a pathway to market; Learn how your company can form a regulatory strategy for your medical device in 8 easy steps. Here’s what to consider when planning one. It involves carefully selecting the best markets and pathways. Medical Device Regulatory Strategy Example.
From www.linkedin.com
HOW GLOBAL REGULATORY STRATEGIES CREATE PATHWAYS FOR MEDICAL DEVICES Medical Device Regulatory Strategy Example It goes beyond simply choosing a pathway to market; Learn how your company can form a regulatory strategy for your medical device in 8 easy steps. It involves carefully selecting the best markets and pathways for both your device and your business. We provide a review of emerging strategies, opportunities, and best practices to increase the regulatory knowledge base and.. Medical Device Regulatory Strategy Example.
From www.slideshare.net
Regulatory Strategies for Medical Device Companies to Succeed in Asia Medical Device Regulatory Strategy Example Your medical device global regulatory strategy can have a huge impact on the future success of your product. It involves carefully selecting the best markets and pathways for both your device and your business. Learn how your company can form a regulatory strategy for your medical device in 8 easy steps. Raps has released the second edition of its global. Medical Device Regulatory Strategy Example.
From www.apcerls.com
Safety & Regulatory requirements for Medical Devices APCER Life Sciences Medical Device Regulatory Strategy Example Here’s what to consider when planning one. We provide a review of emerging strategies, opportunities, and best practices to increase the regulatory knowledge base and. Read our tips for preparing a regulatory strategy for medical devices in the united states (us) and the european union (eu). Your medical device global regulatory strategy can have a huge impact on the future. Medical Device Regulatory Strategy Example.
From www.greenlight.guru
Regulatory Strategy for Medical Devices Global Market Planning Medical Device Regulatory Strategy Example Your medical device global regulatory strategy can have a huge impact on the future success of your product. Here’s what to consider when planning one. We provide a review of emerging strategies, opportunities, and best practices to increase the regulatory knowledge base and. It involves carefully selecting the best markets and pathways for both your device and your business. Read. Medical Device Regulatory Strategy Example.
From blog.sierralabs.com
8 Regulatory Strategy Guidelines for Your Medical Device Medical Device Regulatory Strategy Example Raps has released the second edition of its global medical device regulatory strategy book, providing a @how to@ guide to. Your medical device global regulatory strategy can have a huge impact on the future success of your product. It involves carefully selecting the best markets and pathways for both your device and your business. It goes beyond simply choosing a. Medical Device Regulatory Strategy Example.
From blog.sierralabs.com
8 Regulatory Strategy Guidelines for Your Medical Device Medical Device Regulatory Strategy Example Read our tips for preparing a regulatory strategy for medical devices in the united states (us) and the european union (eu). Raps has released the second edition of its global medical device regulatory strategy book, providing a @how to@ guide to. It involves carefully selecting the best markets and pathways for both your device and your business. It goes beyond. Medical Device Regulatory Strategy Example.
From old.sermitsiaq.ag
Regulatory Strategy Template For Medical Devices Medical Device Regulatory Strategy Example Here’s what to consider when planning one. Learn how your company can form a regulatory strategy for your medical device in 8 easy steps. It involves carefully selecting the best markets and pathways for both your device and your business. Your medical device global regulatory strategy can have a huge impact on the future success of your product. It goes. Medical Device Regulatory Strategy Example.
From www.scribd.com
Global Medical Device Regulatory Strategy Second Edition Sample Chapter Medical Device Regulatory Strategy Example Here’s what to consider when planning one. Learn how your company can form a regulatory strategy for your medical device in 8 easy steps. Read our tips for preparing a regulatory strategy for medical devices in the united states (us) and the european union (eu). We provide a review of emerging strategies, opportunities, and best practices to increase the regulatory. Medical Device Regulatory Strategy Example.
From www.slideshare.net
Regulatory strategy for medical device startups Medical Device Regulatory Strategy Example We provide a review of emerging strategies, opportunities, and best practices to increase the regulatory knowledge base and. Read our tips for preparing a regulatory strategy for medical devices in the united states (us) and the european union (eu). Raps has released the second edition of its global medical device regulatory strategy book, providing a @how to@ guide to. Here’s. Medical Device Regulatory Strategy Example.
From studylib.net
Regulatory Overview Table Medical Device Regulatory Strategy Example It goes beyond simply choosing a pathway to market; Raps has released the second edition of its global medical device regulatory strategy book, providing a @how to@ guide to. Learn how your company can form a regulatory strategy for your medical device in 8 easy steps. It involves carefully selecting the best markets and pathways for both your device and. Medical Device Regulatory Strategy Example.
From www.slideshare.net
Regulatory Strategies for Medical Device Companies to Succeed in Asia Medical Device Regulatory Strategy Example It involves carefully selecting the best markets and pathways for both your device and your business. Here’s what to consider when planning one. We provide a review of emerging strategies, opportunities, and best practices to increase the regulatory knowledge base and. Raps has released the second edition of its global medical device regulatory strategy book, providing a @how to@ guide. Medical Device Regulatory Strategy Example.
From blog.sierralabs.com
ISO 13485 Regulatory Requirements on Medical Devices Medical Device Regulatory Strategy Example It goes beyond simply choosing a pathway to market; Here’s what to consider when planning one. Your medical device global regulatory strategy can have a huge impact on the future success of your product. We provide a review of emerging strategies, opportunities, and best practices to increase the regulatory knowledge base and. It involves carefully selecting the best markets and. Medical Device Regulatory Strategy Example.
From www.hardianhealth.com
Medical Device Regulatory Services UKCA, CE and FDA — Hardian Health Medical Device Regulatory Strategy Example Raps has released the second edition of its global medical device regulatory strategy book, providing a @how to@ guide to. Learn how your company can form a regulatory strategy for your medical device in 8 easy steps. It goes beyond simply choosing a pathway to market; Here’s what to consider when planning one. We provide a review of emerging strategies,. Medical Device Regulatory Strategy Example.
From www.heartlungcirc.org
Regulatory Requirements For Medical Devices And Vascular Ageing An Medical Device Regulatory Strategy Example It goes beyond simply choosing a pathway to market; Learn how your company can form a regulatory strategy for your medical device in 8 easy steps. It involves carefully selecting the best markets and pathways for both your device and your business. Read our tips for preparing a regulatory strategy for medical devices in the united states (us) and the. Medical Device Regulatory Strategy Example.
From exeedqm.com
FDA Guidance Shows a Regulatory Path Forward for Interoperable Devices Medical Device Regulatory Strategy Example Your medical device global regulatory strategy can have a huge impact on the future success of your product. Learn how your company can form a regulatory strategy for your medical device in 8 easy steps. It goes beyond simply choosing a pathway to market; Raps has released the second edition of its global medical device regulatory strategy book, providing a. Medical Device Regulatory Strategy Example.
From sunstonepilot.com
Introduction to Medical Device Development Sunstone Pilot, Inc. Medical Device Regulatory Strategy Example Learn how your company can form a regulatory strategy for your medical device in 8 easy steps. Here’s what to consider when planning one. Your medical device global regulatory strategy can have a huge impact on the future success of your product. We provide a review of emerging strategies, opportunities, and best practices to increase the regulatory knowledge base and.. Medical Device Regulatory Strategy Example.
From www.slideteam.net
Five Years Medical Device Planning Roadmap With FDA Regulatory Medical Device Regulatory Strategy Example Read our tips for preparing a regulatory strategy for medical devices in the united states (us) and the european union (eu). It goes beyond simply choosing a pathway to market; It involves carefully selecting the best markets and pathways for both your device and your business. Here’s what to consider when planning one. Your medical device global regulatory strategy can. Medical Device Regulatory Strategy Example.
From www.slideshare.net
Regulatory Strategies for Medical Device Companies to Succeed in Asia Medical Device Regulatory Strategy Example Raps has released the second edition of its global medical device regulatory strategy book, providing a @how to@ guide to. We provide a review of emerging strategies, opportunities, and best practices to increase the regulatory knowledge base and. Learn how your company can form a regulatory strategy for your medical device in 8 easy steps. Your medical device global regulatory. Medical Device Regulatory Strategy Example.
From template.mapadapalavra.ba.gov.br
Medical Device Regulatory Strategy Template Medical Device Regulatory Strategy Example It involves carefully selecting the best markets and pathways for both your device and your business. Raps has released the second edition of its global medical device regulatory strategy book, providing a @how to@ guide to. Your medical device global regulatory strategy can have a huge impact on the future success of your product. Read our tips for preparing a. Medical Device Regulatory Strategy Example.
From www.slideteam.net
Six Months Medical Device Development Roadmap With FDA Regulatory Medical Device Regulatory Strategy Example Read our tips for preparing a regulatory strategy for medical devices in the united states (us) and the european union (eu). Learn how your company can form a regulatory strategy for your medical device in 8 easy steps. We provide a review of emerging strategies, opportunities, and best practices to increase the regulatory knowledge base and. Raps has released the. Medical Device Regulatory Strategy Example.
From www.youtube.com
Regulatory Standards & Risk Management in Medical Devices YouTube Medical Device Regulatory Strategy Example We provide a review of emerging strategies, opportunities, and best practices to increase the regulatory knowledge base and. Here’s what to consider when planning one. Your medical device global regulatory strategy can have a huge impact on the future success of your product. It goes beyond simply choosing a pathway to market; Raps has released the second edition of its. Medical Device Regulatory Strategy Example.
From old.sermitsiaq.ag
Regulatory Strategy Template For Medical Devices Medical Device Regulatory Strategy Example It involves carefully selecting the best markets and pathways for both your device and your business. Read our tips for preparing a regulatory strategy for medical devices in the united states (us) and the european union (eu). Raps has released the second edition of its global medical device regulatory strategy book, providing a @how to@ guide to. Your medical device. Medical Device Regulatory Strategy Example.
From www.slideteam.net
Six Months Roadmap Of Medical Device Approval From FDA Regulatory Medical Device Regulatory Strategy Example We provide a review of emerging strategies, opportunities, and best practices to increase the regulatory knowledge base and. Learn how your company can form a regulatory strategy for your medical device in 8 easy steps. It goes beyond simply choosing a pathway to market; Here’s what to consider when planning one. Your medical device global regulatory strategy can have a. Medical Device Regulatory Strategy Example.
From mdlaw.eu
MDR Regulatory Strategy implementation guide · MDlaw Information Medical Device Regulatory Strategy Example Raps has released the second edition of its global medical device regulatory strategy book, providing a @how to@ guide to. Here’s what to consider when planning one. We provide a review of emerging strategies, opportunities, and best practices to increase the regulatory knowledge base and. It goes beyond simply choosing a pathway to market; Read our tips for preparing a. Medical Device Regulatory Strategy Example.
From blog.sierralabs.com
6 Regulatory Pathways to Bring Your Medical Device to Market Medical Device Regulatory Strategy Example It goes beyond simply choosing a pathway to market; We provide a review of emerging strategies, opportunities, and best practices to increase the regulatory knowledge base and. It involves carefully selecting the best markets and pathways for both your device and your business. Raps has released the second edition of its global medical device regulatory strategy book, providing a @how. Medical Device Regulatory Strategy Example.
From planetinnovation.com
The 4 key principles of a smart regulatory strategy for medical devices Medical Device Regulatory Strategy Example Raps has released the second edition of its global medical device regulatory strategy book, providing a @how to@ guide to. We provide a review of emerging strategies, opportunities, and best practices to increase the regulatory knowledge base and. It involves carefully selecting the best markets and pathways for both your device and your business. Read our tips for preparing a. Medical Device Regulatory Strategy Example.
From www.mcra.com
Medical Device FDA Regulatory Consulting MCRA Medical Device Regulatory Strategy Example We provide a review of emerging strategies, opportunities, and best practices to increase the regulatory knowledge base and. Here’s what to consider when planning one. Raps has released the second edition of its global medical device regulatory strategy book, providing a @how to@ guide to. Your medical device global regulatory strategy can have a huge impact on the future success. Medical Device Regulatory Strategy Example.
From www.researchgate.net
The schematic summary of the regulatory submissions and approval Medical Device Regulatory Strategy Example Raps has released the second edition of its global medical device regulatory strategy book, providing a @how to@ guide to. Your medical device global regulatory strategy can have a huge impact on the future success of your product. It involves carefully selecting the best markets and pathways for both your device and your business. Learn how your company can form. Medical Device Regulatory Strategy Example.
From data1.skinnyms.com
Regulatory Strategy Template For Medical Devices Medical Device Regulatory Strategy Example It involves carefully selecting the best markets and pathways for both your device and your business. Here’s what to consider when planning one. Read our tips for preparing a regulatory strategy for medical devices in the united states (us) and the european union (eu). We provide a review of emerging strategies, opportunities, and best practices to increase the regulatory knowledge. Medical Device Regulatory Strategy Example.
From www.greenlight.guru
Understanding the 5 Phases of Medical Device Development Medical Device Regulatory Strategy Example Your medical device global regulatory strategy can have a huge impact on the future success of your product. Learn how your company can form a regulatory strategy for your medical device in 8 easy steps. Raps has released the second edition of its global medical device regulatory strategy book, providing a @how to@ guide to. It involves carefully selecting the. Medical Device Regulatory Strategy Example.