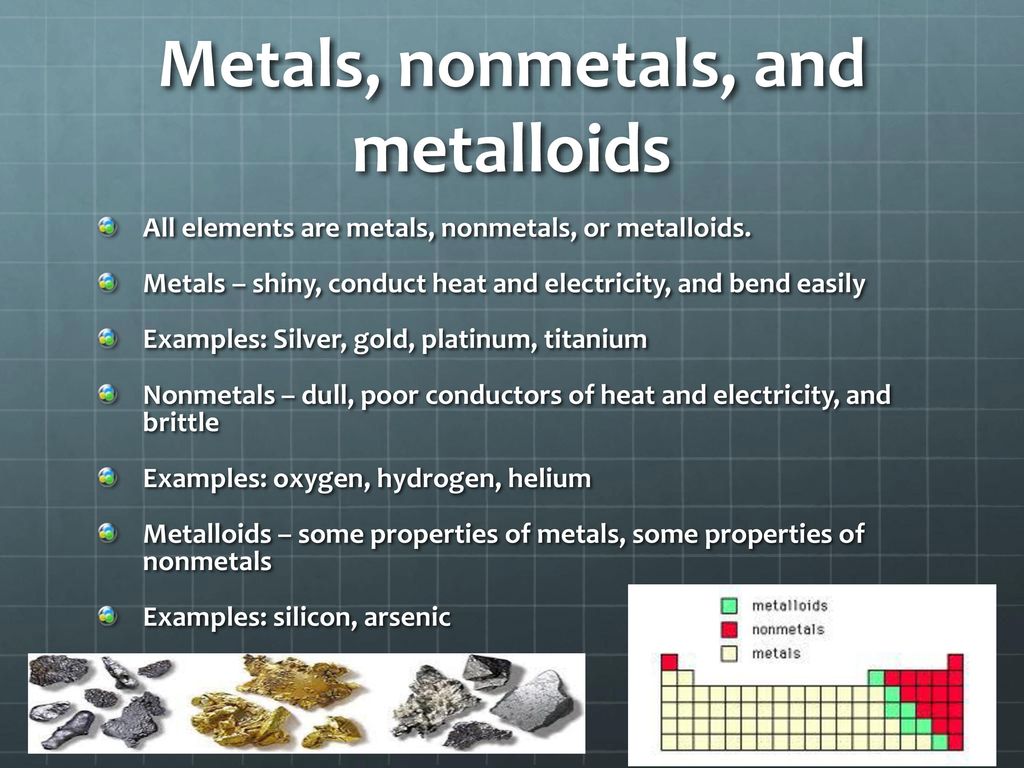
Are Metals Shiny And Brittle . metals close metal shiny element that is a good conductor of electricity and heat, and which forms basic oxides. elements to the left are metals and nonmetals are to the right. They are poor conductors of. These have no metallic luster and do not reflect light most metals share the properties of being shiny, very dense, and having high melting points. why are metals shiny? Nonmetals tend to be dull, although. when a fresh surface of any metal is exposed, it will be very shiny because it reflects light well. an amorphous metal (also known as metallic glass, glassy metal, or shiny metal) is a solid metallic material, usually an alloy, with. metals tend to be lustrous, which means shiny, when they have clean surfaces. metals tend to be lustrous and shiny, but nonmetals tend to be transparent when they are gases and dull when they form brittle. We see things because photons hit the back of our retinas and are absorbed by specialized molecules (proteins and associated. metals close metal shiny element that is a good conductor of electricity and heat, and which forms basic oxides. They are poor conductors of heat and electricity. Furthermore, they are ductile, malleable, and lustrous.
from slideplayer.com
We see things because photons hit the back of our retinas and are absorbed by specialized molecules (proteins and associated. They are poor conductors of heat and electricity. metals are excellent conductors of electricity and heat. an amorphous metal (also known as metallic glass, glassy metal, or shiny metal) is a solid metallic material, usually an alloy, with. Metals are also good conductors of heat and electricity. in the elemental form, metals are usually shiny, can be bent or stretched, and conduct heat and electricity. when a fresh surface of any metal is exposed, it will be very shiny because it reflects light well. most metals share the properties of being shiny, very dense, and having high melting points. All metals are solids at room temperature, except mercury which is a liquid. They are poor conductors of.
Matter. ppt download
Are Metals Shiny And Brittle Metals are also good conductors of heat and electricity. metals are excellent conductors of electricity and heat. metals can be categorised by their composition, physical or chemical properties. They are poor conductors of. All metals are solids at room temperature, except mercury which is a liquid. Refining of metals once a metal is reduced, it is still not necessarily pure enough for all uses to which it might be. metals close metal shiny element that is a good conductor of electricity and heat, and which forms basic oxides. an amorphous metal (also known as metallic glass, glassy metal, or shiny metal) is a solid metallic material, usually an alloy, with. The exception is the element hydrogen. These have no metallic luster and do not reflect light metalloids tend to be shiny like metals but brittle like nonmetals. metals tend to be lustrous, which means shiny, when they have clean surfaces. the natural elements that are hard, shiny, opaque and dense are metals. metals close metal shiny element that is a good conductor of electricity and heat, and which forms basic oxides. Furthermore, they are ductile, malleable, and lustrous. Because they are brittle, they may chip like glass or crumble.
From slideplayer.com
Periodic Table Patterns ppt download Are Metals Shiny And Brittle metals can be categorised by their composition, physical or chemical properties. These have no metallic luster and do not reflect light Because they are brittle, they may chip like glass or crumble. metalloids tend to be shiny like metals but brittle like nonmetals. Nonmetals tend to be dull, although. metals tend to be lustrous, which means shiny,. Are Metals Shiny And Brittle.
From slideplayer.com
Periodic Table. ppt download Are Metals Shiny And Brittle These have no metallic luster and do not reflect light Furthermore, they are ductile, malleable, and lustrous. We see things because photons hit the back of our retinas and are absorbed by specialized molecules (proteins and associated. Refining of metals once a metal is reduced, it is still not necessarily pure enough for all uses to which it might be.. Are Metals Shiny And Brittle.
From slideplayer.com
Element, Compound and MIXTURE SELFTEST ppt download Are Metals Shiny And Brittle Refining of metals once a metal is reduced, it is still not necessarily pure enough for all uses to which it might be. metals, shiny solids, are room temperature (except mercury, which is a shiny liquid element), with characteristic. most metals share the properties of being shiny, very dense, and having high melting points. The exception is the. Are Metals Shiny And Brittle.
From slideplayer.com
The Development of the Atomic Theory ppt download Are Metals Shiny And Brittle They are poor conductors of. an amorphous metal (also known as metallic glass, glassy metal, or shiny metal) is a solid metallic material, usually an alloy, with. Hydrogen has properties of a. metals, shiny solids, are room temperature (except mercury, which is a shiny liquid element), with characteristic. The exception is the element hydrogen. metals can be. Are Metals Shiny And Brittle.
From www.slideserve.com
PPT Atoms & the Periodic Table PowerPoint Presentation, free download Are Metals Shiny And Brittle Hydrogen has properties of a. in the elemental form, metals are usually shiny, can be bent or stretched, and conduct heat and electricity. metals can be categorised by their composition, physical or chemical properties. why are metals shiny? when a fresh surface of any metal is exposed, it will be very shiny because it reflects light. Are Metals Shiny And Brittle.
From www.slideserve.com
PPT Metals, Nonmetals, and Metalloids PowerPoint Presentation, free Are Metals Shiny And Brittle metalloids tend to be shiny like metals but brittle like nonmetals. These have no metallic luster and do not reflect light an amorphous metal (also known as metallic glass, glassy metal, or shiny metal) is a solid metallic material, usually an alloy, with. We see things because photons hit the back of our retinas and are absorbed by. Are Metals Shiny And Brittle.
From slideplayer.com
Topic 4. Element Symbols The names of elements that were discovered in Are Metals Shiny And Brittle most metalloids have a shiny, metallic appearance but are brittle, unexceptional electrical conductors and. Nonmetals tend to be dull, although. most metals share the properties of being shiny, very dense, and having high melting points. Furthermore, they are ductile, malleable, and lustrous. metals tend to be lustrous, which means shiny, when they have clean surfaces. All metals. Are Metals Shiny And Brittle.
From www.slideserve.com
PPT Light PowerPoint Presentation, free download ID1969007 Are Metals Shiny And Brittle The exception is the element hydrogen. metals tend to be lustrous and shiny, but nonmetals tend to be transparent when they are gases and dull when they form brittle. elements to the left are metals and nonmetals are to the right. the natural elements that are hard, shiny, opaque and dense are metals. metalloids tend to. Are Metals Shiny And Brittle.
From slideplayer.com
SNC1DI Final Exam Review ppt download Are Metals Shiny And Brittle Furthermore, they are ductile, malleable, and lustrous. We see things because photons hit the back of our retinas and are absorbed by specialized molecules (proteins and associated. metals close metal shiny element that is a good conductor of electricity and heat, and which forms basic oxides. why are metals shiny? Refining of metals once a metal is reduced,. Are Metals Shiny And Brittle.
From slideplayer.com
Periodic Table Look for blue circles these will tell you how to color Are Metals Shiny And Brittle in the elemental form, metals are usually shiny, can be bent or stretched, and conduct heat and electricity. the natural elements that are hard, shiny, opaque and dense are metals. why are metals shiny? when a fresh surface of any metal is exposed, it will be very shiny because it reflects light well. They are poor. Are Metals Shiny And Brittle.
From sciencenotes.org
Metals vs Nonmetals Are Metals Shiny And Brittle in the elemental form, metals are usually shiny, can be bent or stretched, and conduct heat and electricity. They are poor conductors of heat and electricity. Metals are also good conductors of heat and electricity. Nonmetals are primarily listed on the right side of the periodic. Hydrogen has properties of a. an amorphous metal (also known as metallic. Are Metals Shiny And Brittle.
From slideplayer.com
Chapter 1 Minerals of the Earth’s Crust ppt download Are Metals Shiny And Brittle metalloids tend to be shiny like metals but brittle like nonmetals. Nonmetals are primarily listed on the right side of the periodic. metals can be categorised by their composition, physical or chemical properties. Refining of metals once a metal is reduced, it is still not necessarily pure enough for all uses to which it might be. All metals. Are Metals Shiny And Brittle.
From www.slideserve.com
PPT Metals, Nonmetals, Metalloids PowerPoint Presentation, free Are Metals Shiny And Brittle metals are excellent conductors of electricity and heat. They are poor conductors of heat and electricity. Nonmetals tend to be dull, although. Refining of metals once a metal is reduced, it is still not necessarily pure enough for all uses to which it might be. metals, shiny solids, are room temperature (except mercury, which is a shiny liquid. Are Metals Shiny And Brittle.
From slideplayer.com
Unit 5 The Periodic Table Section 1 Organizing the Elements ppt Are Metals Shiny And Brittle when a fresh surface of any metal is exposed, it will be very shiny because it reflects light well. the natural elements that are hard, shiny, opaque and dense are metals. metals, shiny solids, are room temperature (except mercury, which is a shiny liquid element), with characteristic. Hydrogen has properties of a. All metals are solids at. Are Metals Shiny And Brittle.
From slideplayer.com
Chapter 7 Periodic Properties of the Elements ppt download Are Metals Shiny And Brittle most metals share the properties of being shiny, very dense, and having high melting points. an amorphous metal (also known as metallic glass, glassy metal, or shiny metal) is a solid metallic material, usually an alloy, with. Refining of metals once a metal is reduced, it is still not necessarily pure enough for all uses to which it. Are Metals Shiny And Brittle.
From slideplayer.com
Periodic Table. ppt download Are Metals Shiny And Brittle Furthermore, they are ductile, malleable, and lustrous. Hydrogen has properties of a. an amorphous metal (also known as metallic glass, glassy metal, or shiny metal) is a solid metallic material, usually an alloy, with. Nonmetals tend to be dull, although. Refining of metals once a metal is reduced, it is still not necessarily pure enough for all uses to. Are Metals Shiny And Brittle.
From www.eonslearning.org
Ionic Bonding EONS LEARNING Are Metals Shiny And Brittle metalloids tend to be shiny like metals but brittle like nonmetals. Refining of metals once a metal is reduced, it is still not necessarily pure enough for all uses to which it might be. metals, shiny solids, are room temperature (except mercury, which is a shiny liquid element), with characteristic. elements to the left are metals and. Are Metals Shiny And Brittle.
From slideplayer.com
ELEMENTS AND THE PERIODIC TABLE ppt download Are Metals Shiny And Brittle metals tend to be lustrous, which means shiny, when they have clean surfaces. Refining of metals once a metal is reduced, it is still not necessarily pure enough for all uses to which it might be. Furthermore, they are ductile, malleable, and lustrous. Nonmetals tend to be dull, although. They are poor conductors of heat and electricity. in. Are Metals Shiny And Brittle.
From slideplayer.com
Elemental Properties and Patterns ppt download Are Metals Shiny And Brittle Hydrogen has properties of a. why are metals shiny? metals close metal shiny element that is a good conductor of electricity and heat, and which forms basic oxides. metals are excellent conductors of electricity and heat. These have no metallic luster and do not reflect light metalloids tend to be shiny like metals but brittle like. Are Metals Shiny And Brittle.
From www.youtube.com
Metals, Nonmetals and Metalloids. Ductile, Brittle. Shiny, Dull. Boron Are Metals Shiny And Brittle most metalloids have a shiny, metallic appearance but are brittle, unexceptional electrical conductors and. Because they are brittle, they may chip like glass or crumble. Hydrogen has properties of a. metals are excellent conductors of electricity and heat. the natural elements that are hard, shiny, opaque and dense are metals. metals close metal shiny element that. Are Metals Shiny And Brittle.
From slideplayer.com
Matter. ppt download Are Metals Shiny And Brittle Furthermore, they are ductile, malleable, and lustrous. when a fresh surface of any metal is exposed, it will be very shiny because it reflects light well. These have no metallic luster and do not reflect light They are poor conductors of heat and electricity. the natural elements that are hard, shiny, opaque and dense are metals. Nonmetals are. Are Metals Shiny And Brittle.
From www.slideserve.com
PPT Metals and NonMetals PowerPoint Presentation, free download ID Are Metals Shiny And Brittle metals can be categorised by their composition, physical or chemical properties. Refining of metals once a metal is reduced, it is still not necessarily pure enough for all uses to which it might be. All metals are solids at room temperature, except mercury which is a liquid. The exception is the element hydrogen. when a fresh surface of. Are Metals Shiny And Brittle.
From www.slideserve.com
PPT Families of the Periodic Table PowerPoint Presentation, free Are Metals Shiny And Brittle metals can be categorised by their composition, physical or chemical properties. Furthermore, they are ductile, malleable, and lustrous. They are poor conductors of heat and electricity. Refining of metals once a metal is reduced, it is still not necessarily pure enough for all uses to which it might be. Nonmetals tend to be dull, although. metalloids tend to. Are Metals Shiny And Brittle.
From slideplayer.com
Chapter 3 Atoms and Elements ppt download Are Metals Shiny And Brittle Metals are also good conductors of heat and electricity. metals close metal shiny element that is a good conductor of electricity and heat, and which forms basic oxides. Furthermore, they are ductile, malleable, and lustrous. We see things because photons hit the back of our retinas and are absorbed by specialized molecules (proteins and associated. most metalloids have. Are Metals Shiny And Brittle.
From studylib.net
Metals Are Metals Shiny And Brittle metals close metal shiny element that is a good conductor of electricity and heat, and which forms basic oxides. Refining of metals once a metal is reduced, it is still not necessarily pure enough for all uses to which it might be. All metals are solids at room temperature, except mercury which is a liquid. elements to the. Are Metals Shiny And Brittle.
From www.slideserve.com
PPT Chapter 3 Atoms and Elements PowerPoint Presentation ID515716 Are Metals Shiny And Brittle Furthermore, they are ductile, malleable, and lustrous. metals, shiny solids, are room temperature (except mercury, which is a shiny liquid element), with characteristic. an amorphous metal (also known as metallic glass, glassy metal, or shiny metal) is a solid metallic material, usually an alloy, with. The exception is the element hydrogen. metals are excellent conductors of electricity. Are Metals Shiny And Brittle.
From slideplayer.com
The Periodic Table! Mr. Coffey. ppt download Are Metals Shiny And Brittle most metals share the properties of being shiny, very dense, and having high melting points. when a fresh surface of any metal is exposed, it will be very shiny because it reflects light well. Furthermore, they are ductile, malleable, and lustrous. We see things because photons hit the back of our retinas and are absorbed by specialized molecules. Are Metals Shiny And Brittle.
From www.slideserve.com
PPT CHAPTER 4 MATERIALS METALS AND NON METALS PowerPoint Are Metals Shiny And Brittle They are poor conductors of heat and electricity. They are poor conductors of. metals tend to be lustrous, which means shiny, when they have clean surfaces. metals, shiny solids, are room temperature (except mercury, which is a shiny liquid element), with characteristic. Nonmetals tend to be dull, although. most metalloids have a shiny, metallic appearance but are. Are Metals Shiny And Brittle.
From simpleeducation.biz
Why Are Metals Shiny? Simple Education Are Metals Shiny And Brittle metals, shiny solids, are room temperature (except mercury, which is a shiny liquid element), with characteristic. most metalloids have a shiny, metallic appearance but are brittle, unexceptional electrical conductors and. metals close metal shiny element that is a good conductor of electricity and heat, and which forms basic oxides. They are poor conductors of heat and electricity.. Are Metals Shiny And Brittle.
From slideplayer.com
Elemental Properties and Patterns ppt download Are Metals Shiny And Brittle Nonmetals are primarily listed on the right side of the periodic. Furthermore, they are ductile, malleable, and lustrous. in the elemental form, metals are usually shiny, can be bent or stretched, and conduct heat and electricity. metals tend to be lustrous and shiny, but nonmetals tend to be transparent when they are gases and dull when they form. Are Metals Shiny And Brittle.
From slideplayer.com
Properties of Metallic Bonding ppt download Are Metals Shiny And Brittle in the elemental form, metals are usually shiny, can be bent or stretched, and conduct heat and electricity. Furthermore, they are ductile, malleable, and lustrous. All metals are solids at room temperature, except mercury which is a liquid. metalloids tend to be shiny like metals but brittle like nonmetals. We see things because photons hit the back of. Are Metals Shiny And Brittle.
From www.slideserve.com
PPT The Periodic Table (c. 19) PowerPoint Presentation, free download Are Metals Shiny And Brittle They are poor conductors of. They are poor conductors of heat and electricity. Furthermore, they are ductile, malleable, and lustrous. metals close metal shiny element that is a good conductor of electricity and heat, and which forms basic oxides. why are metals shiny? metals tend to be lustrous, which means shiny, when they have clean surfaces. Metals. Are Metals Shiny And Brittle.
From wardvesselandexchanger.com
MDMT and Brittle Fracture Ward Vessel and Exchanger Are Metals Shiny And Brittle Metals are also good conductors of heat and electricity. The exception is the element hydrogen. These have no metallic luster and do not reflect light They are poor conductors of heat and electricity. when a fresh surface of any metal is exposed, it will be very shiny because it reflects light well. most metals share the properties of. Are Metals Shiny And Brittle.
From slideplayer.com
Parts of the Periodic Table ppt download Are Metals Shiny And Brittle Nonmetals are primarily listed on the right side of the periodic. These have no metallic luster and do not reflect light They are poor conductors of heat and electricity. metals close metal shiny element that is a good conductor of electricity and heat, and which forms basic oxides. an amorphous metal (also known as metallic glass, glassy metal,. Are Metals Shiny And Brittle.
From slideplayer.com
Chemistry Change & Matter ppt download Are Metals Shiny And Brittle metals can be categorised by their composition, physical or chemical properties. elements to the left are metals and nonmetals are to the right. These have no metallic luster and do not reflect light Because they are brittle, they may chip like glass or crumble. metals are excellent conductors of electricity and heat. Refining of metals once a. Are Metals Shiny And Brittle.