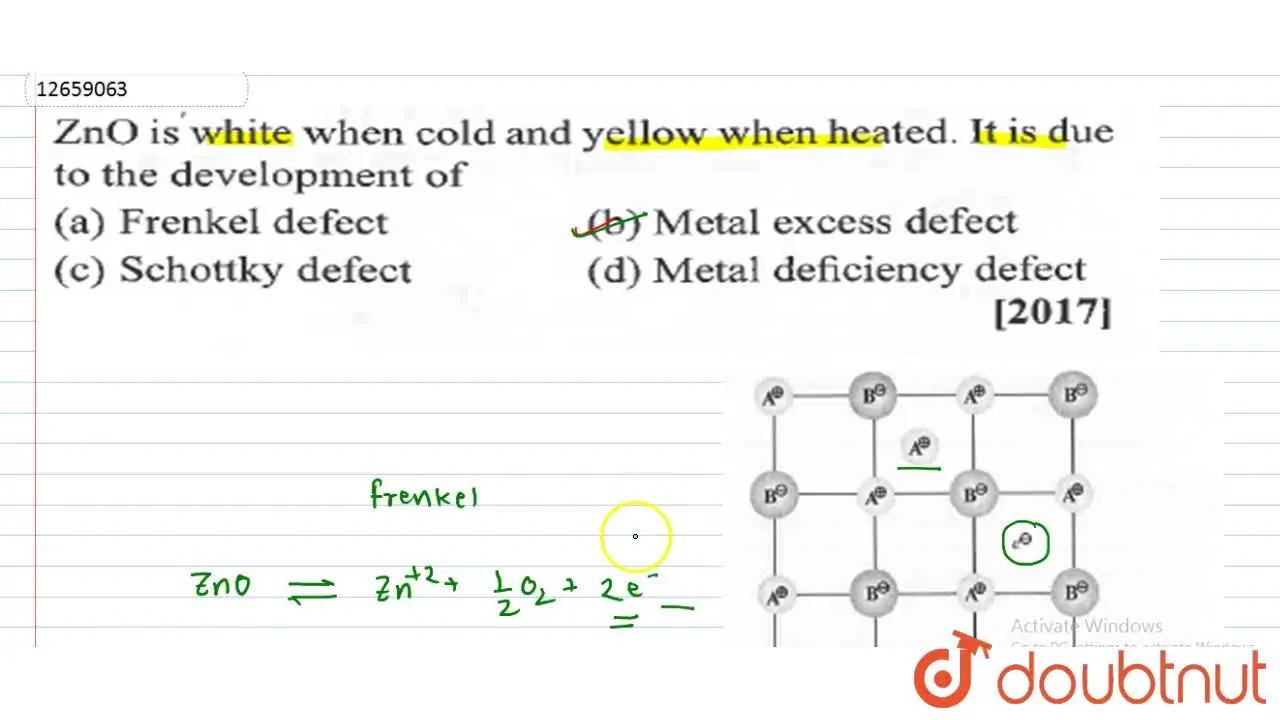
Why Does White Zno (S) Becomes Yellow Upon Heating . On heating, zno gives , electrons and colour. Why does white zno(s) become yellow upon heating? After cooling down, the colour of zinc oxide changes back to. The zno crystal becomes yellow oh heating because of the metal excess defect which is caused due to the presence of extra cations at. On heating zno, it loses oxygen and electrons, as shown below: The excess zn ions thus formed move to the interstitial sites and electron in the. Zinc oxide, white in colour at room temperature, acquires yellow colour on heating due to: On heating zno loses oxygen according to the following reaction. In $\ce{zno}$, $\ce{zn}$ is present in the 2+ oxidation state, and the d subshell has 10 electron, and the s subshell has 0. When zinc oxide is heated strongly above ${800^ \circ }{\text{c}}$ it turns yellow. Zn 2+ ions and electrons move to interstitial sites and f. Why does white zn0(s) becomes yellow upon heating ?
from www.doubtnut.com
In $\ce{zno}$, $\ce{zn}$ is present in the 2+ oxidation state, and the d subshell has 10 electron, and the s subshell has 0. Why does white zn0(s) becomes yellow upon heating ? On heating zno loses oxygen according to the following reaction. The excess zn ions thus formed move to the interstitial sites and electron in the. Zn 2+ ions and electrons move to interstitial sites and f. The zno crystal becomes yellow oh heating because of the metal excess defect which is caused due to the presence of extra cations at. Why does white zno(s) become yellow upon heating? When zinc oxide is heated strongly above ${800^ \circ }{\text{c}}$ it turns yellow. After cooling down, the colour of zinc oxide changes back to. On heating, zno gives , electrons and colour.
ZnO is white cold and yellow when heated, it is due to the development
Why Does White Zno (S) Becomes Yellow Upon Heating On heating, zno gives , electrons and colour. On heating zno, it loses oxygen and electrons, as shown below: Why does white zn0(s) becomes yellow upon heating ? In $\ce{zno}$, $\ce{zn}$ is present in the 2+ oxidation state, and the d subshell has 10 electron, and the s subshell has 0. On heating zno loses oxygen according to the following reaction. The excess zn ions thus formed move to the interstitial sites and electron in the. The zno crystal becomes yellow oh heating because of the metal excess defect which is caused due to the presence of extra cations at. Zinc oxide, white in colour at room temperature, acquires yellow colour on heating due to: Zn 2+ ions and electrons move to interstitial sites and f. On heating, zno gives , electrons and colour. After cooling down, the colour of zinc oxide changes back to. When zinc oxide is heated strongly above ${800^ \circ }{\text{c}}$ it turns yellow. Why does white zno(s) become yellow upon heating?
From www.doubtnut.com
ZnO shows yellow colour on heating due to Why Does White Zno (S) Becomes Yellow Upon Heating On heating zno loses oxygen according to the following reaction. The excess zn ions thus formed move to the interstitial sites and electron in the. Zn 2+ ions and electrons move to interstitial sites and f. On heating, zno gives , electrons and colour. When zinc oxide is heated strongly above ${800^ \circ }{\text{c}}$ it turns yellow. Why does white. Why Does White Zno (S) Becomes Yellow Upon Heating.
From www.researchgate.net
Micrographs of heattreated ZnO ceramics at 800°C/2h with compositions Why Does White Zno (S) Becomes Yellow Upon Heating After cooling down, the colour of zinc oxide changes back to. Why does white zn0(s) becomes yellow upon heating ? The excess zn ions thus formed move to the interstitial sites and electron in the. Zn 2+ ions and electrons move to interstitial sites and f. Why does white zno(s) become yellow upon heating? Zinc oxide, white in colour at. Why Does White Zno (S) Becomes Yellow Upon Heating.
From www.youtube.com
Why does white zinc oxide on heating yellow ? 12 THE SOLID Why Does White Zno (S) Becomes Yellow Upon Heating In $\ce{zno}$, $\ce{zn}$ is present in the 2+ oxidation state, and the d subshell has 10 electron, and the s subshell has 0. When zinc oxide is heated strongly above ${800^ \circ }{\text{c}}$ it turns yellow. The zno crystal becomes yellow oh heating because of the metal excess defect which is caused due to the presence of extra cations at.. Why Does White Zno (S) Becomes Yellow Upon Heating.
From www.slideshare.net
Action of heat on chemical compound Why Does White Zno (S) Becomes Yellow Upon Heating The excess zn ions thus formed move to the interstitial sites and electron in the. After cooling down, the colour of zinc oxide changes back to. Why does white zno(s) become yellow upon heating? On heating zno loses oxygen according to the following reaction. In $\ce{zno}$, $\ce{zn}$ is present in the 2+ oxidation state, and the d subshell has 10. Why Does White Zno (S) Becomes Yellow Upon Heating.
From www.doubtnut.com
When ZnO is heated it turns yellow and returns back to original white Why Does White Zno (S) Becomes Yellow Upon Heating The zno crystal becomes yellow oh heating because of the metal excess defect which is caused due to the presence of extra cations at. On heating zno, it loses oxygen and electrons, as shown below: On heating zno loses oxygen according to the following reaction. When zinc oxide is heated strongly above ${800^ \circ }{\text{c}}$ it turns yellow. The excess. Why Does White Zno (S) Becomes Yellow Upon Heating.
From fphoto.photoshelter.com
science chemistry compound zinc oxide Fundamental Photographs The Why Does White Zno (S) Becomes Yellow Upon Heating Why does white zn0(s) becomes yellow upon heating ? On heating, zno gives , electrons and colour. Why does white zno(s) become yellow upon heating? Zn 2+ ions and electrons move to interstitial sites and f. After cooling down, the colour of zinc oxide changes back to. Zinc oxide, white in colour at room temperature, acquires yellow colour on heating. Why Does White Zno (S) Becomes Yellow Upon Heating.
From www.toppr.com
The yellow colour or ZnO and conducting nature produced in heating is Why Does White Zno (S) Becomes Yellow Upon Heating After cooling down, the colour of zinc oxide changes back to. The excess zn ions thus formed move to the interstitial sites and electron in the. In $\ce{zno}$, $\ce{zn}$ is present in the 2+ oxidation state, and the d subshell has 10 electron, and the s subshell has 0. On heating zno loses oxygen according to the following reaction. On. Why Does White Zno (S) Becomes Yellow Upon Heating.
From questions-in.kunduz.com
= 36. The yellow colour of ZnO and condu... Chemistry Why Does White Zno (S) Becomes Yellow Upon Heating On heating, zno gives , electrons and colour. Why does white zn0(s) becomes yellow upon heating ? When zinc oxide is heated strongly above ${800^ \circ }{\text{c}}$ it turns yellow. In $\ce{zno}$, $\ce{zn}$ is present in the 2+ oxidation state, and the d subshell has 10 electron, and the s subshell has 0. The zno crystal becomes yellow oh heating. Why Does White Zno (S) Becomes Yellow Upon Heating.
From www.doubtnut.com
Why does white zinc oxide on heating yellow Why Does White Zno (S) Becomes Yellow Upon Heating Zn 2+ ions and electrons move to interstitial sites and f. After cooling down, the colour of zinc oxide changes back to. Why does white zn0(s) becomes yellow upon heating ? Why does white zno(s) become yellow upon heating? In $\ce{zno}$, $\ce{zn}$ is present in the 2+ oxidation state, and the d subshell has 10 electron, and the s subshell. Why Does White Zno (S) Becomes Yellow Upon Heating.
From www.researchgate.net
a Color change and formation of white precipitate, b mechanism of Why Does White Zno (S) Becomes Yellow Upon Heating On heating, zno gives , electrons and colour. The excess zn ions thus formed move to the interstitial sites and electron in the. In $\ce{zno}$, $\ce{zn}$ is present in the 2+ oxidation state, and the d subshell has 10 electron, and the s subshell has 0. Zn 2+ ions and electrons move to interstitial sites and f. When zinc oxide. Why Does White Zno (S) Becomes Yellow Upon Heating.
From www.youtube.com
Colorchanging Zinc Oxide and submit your questions for Ben YouTube Why Does White Zno (S) Becomes Yellow Upon Heating The zno crystal becomes yellow oh heating because of the metal excess defect which is caused due to the presence of extra cations at. When zinc oxide is heated strongly above ${800^ \circ }{\text{c}}$ it turns yellow. On heating zno loses oxygen according to the following reaction. After cooling down, the colour of zinc oxide changes back to. On heating,. Why Does White Zno (S) Becomes Yellow Upon Heating.
From byjus.com
why ZnO is yellow when hot and white whn cold? Why Does White Zno (S) Becomes Yellow Upon Heating The excess zn ions thus formed move to the interstitial sites and electron in the. In $\ce{zno}$, $\ce{zn}$ is present in the 2+ oxidation state, and the d subshell has 10 electron, and the s subshell has 0. Zinc oxide, white in colour at room temperature, acquires yellow colour on heating due to: On heating zno loses oxygen according to. Why Does White Zno (S) Becomes Yellow Upon Heating.
From www.researchgate.net
Schematic of the synthesis process for the white light R,ZnOZIF8WL Why Does White Zno (S) Becomes Yellow Upon Heating On heating zno loses oxygen according to the following reaction. When zinc oxide is heated strongly above ${800^ \circ }{\text{c}}$ it turns yellow. In $\ce{zno}$, $\ce{zn}$ is present in the 2+ oxidation state, and the d subshell has 10 electron, and the s subshell has 0. On heating, zno gives , electrons and colour. Zinc oxide, white in colour at. Why Does White Zno (S) Becomes Yellow Upon Heating.
From askfilo.com
ZnO is white in colour at room temperature on heating it turns yellow due.. Why Does White Zno (S) Becomes Yellow Upon Heating On heating zno loses oxygen according to the following reaction. Why does white zno(s) become yellow upon heating? After cooling down, the colour of zinc oxide changes back to. The excess zn ions thus formed move to the interstitial sites and electron in the. The zno crystal becomes yellow oh heating because of the metal excess defect which is caused. Why Does White Zno (S) Becomes Yellow Upon Heating.
From www.mdpi.com
Materials Free FullText White Light Emission from ThinFilm Why Does White Zno (S) Becomes Yellow Upon Heating The excess zn ions thus formed move to the interstitial sites and electron in the. In $\ce{zno}$, $\ce{zn}$ is present in the 2+ oxidation state, and the d subshell has 10 electron, and the s subshell has 0. Zinc oxide, white in colour at room temperature, acquires yellow colour on heating due to: After cooling down, the colour of zinc. Why Does White Zno (S) Becomes Yellow Upon Heating.
From www.slideserve.com
PPT Ch 17. J.C. Rowe PowerPoint Presentation ID3099881 Why Does White Zno (S) Becomes Yellow Upon Heating On heating zno, it loses oxygen and electrons, as shown below: The zno crystal becomes yellow oh heating because of the metal excess defect which is caused due to the presence of extra cations at. Why does white zno(s) become yellow upon heating? The excess zn ions thus formed move to the interstitial sites and electron in the. Why does. Why Does White Zno (S) Becomes Yellow Upon Heating.
From www.researchgate.net
Illustration of the structure of ZnO. The yellow and blue spheres Why Does White Zno (S) Becomes Yellow Upon Heating Why does white zn0(s) becomes yellow upon heating ? Why does white zno(s) become yellow upon heating? In $\ce{zno}$, $\ce{zn}$ is present in the 2+ oxidation state, and the d subshell has 10 electron, and the s subshell has 0. After cooling down, the colour of zinc oxide changes back to. On heating zno, it loses oxygen and electrons, as. Why Does White Zno (S) Becomes Yellow Upon Heating.
From fphoto.photoshelter.com
science chemistry compound zinc oxide Fundamental Photographs The Why Does White Zno (S) Becomes Yellow Upon Heating In $\ce{zno}$, $\ce{zn}$ is present in the 2+ oxidation state, and the d subshell has 10 electron, and the s subshell has 0. The excess zn ions thus formed move to the interstitial sites and electron in the. Zinc oxide, white in colour at room temperature, acquires yellow colour on heating due to: On heating zno loses oxygen according to. Why Does White Zno (S) Becomes Yellow Upon Heating.
From www.youtube.com
Why doeshite ZnO (s) yellow upon heating? YouTube Why Does White Zno (S) Becomes Yellow Upon Heating Why does white zno(s) become yellow upon heating? After cooling down, the colour of zinc oxide changes back to. The excess zn ions thus formed move to the interstitial sites and electron in the. The zno crystal becomes yellow oh heating because of the metal excess defect which is caused due to the presence of extra cations at. In $\ce{zno}$,. Why Does White Zno (S) Becomes Yellow Upon Heating.
From www.researchgate.net
(a) Schematic diagram of the yellow (left) and white (right) tandem Why Does White Zno (S) Becomes Yellow Upon Heating On heating, zno gives , electrons and colour. Zn 2+ ions and electrons move to interstitial sites and f. Why does white zn0(s) becomes yellow upon heating ? When zinc oxide is heated strongly above ${800^ \circ }{\text{c}}$ it turns yellow. The excess zn ions thus formed move to the interstitial sites and electron in the. After cooling down, the. Why Does White Zno (S) Becomes Yellow Upon Heating.
From www.mdpi.com
Crystals Free FullText ZnO as a Functional Material, a Review Why Does White Zno (S) Becomes Yellow Upon Heating On heating zno, it loses oxygen and electrons, as shown below: The excess zn ions thus formed move to the interstitial sites and electron in the. Why does white zn0(s) becomes yellow upon heating ? On heating, zno gives , electrons and colour. Zinc oxide, white in colour at room temperature, acquires yellow colour on heating due to: On heating. Why Does White Zno (S) Becomes Yellow Upon Heating.
From slideplayer.com
Organic Ingredients The Basics ppt download Why Does White Zno (S) Becomes Yellow Upon Heating When zinc oxide is heated strongly above ${800^ \circ }{\text{c}}$ it turns yellow. On heating zno loses oxygen according to the following reaction. Why does white zn0(s) becomes yellow upon heating ? Zn 2+ ions and electrons move to interstitial sites and f. Why does white zno(s) become yellow upon heating? On heating, zno gives , electrons and colour. The. Why Does White Zno (S) Becomes Yellow Upon Heating.
From www.doubtnut.com
why does white Zn0(s) yellow upon heating Why Does White Zno (S) Becomes Yellow Upon Heating The excess zn ions thus formed move to the interstitial sites and electron in the. On heating zno, it loses oxygen and electrons, as shown below: Zinc oxide, white in colour at room temperature, acquires yellow colour on heating due to: Zn 2+ ions and electrons move to interstitial sites and f. The zno crystal becomes yellow oh heating because. Why Does White Zno (S) Becomes Yellow Upon Heating.
From www.researchgate.net
The HOMO and LUMO of ZnO and CdZnO. In these examples excited electron Why Does White Zno (S) Becomes Yellow Upon Heating The zno crystal becomes yellow oh heating because of the metal excess defect which is caused due to the presence of extra cations at. In $\ce{zno}$, $\ce{zn}$ is present in the 2+ oxidation state, and the d subshell has 10 electron, and the s subshell has 0. On heating zno loses oxygen according to the following reaction. Zn 2+ ions. Why Does White Zno (S) Becomes Yellow Upon Heating.
From www.doubtnut.com
[Bengali] Why does white ZnO(s) yellow upon heating? Why Does White Zno (S) Becomes Yellow Upon Heating Why does white zno(s) become yellow upon heating? After cooling down, the colour of zinc oxide changes back to. The zno crystal becomes yellow oh heating because of the metal excess defect which is caused due to the presence of extra cations at. On heating zno loses oxygen according to the following reaction. Zn 2+ ions and electrons move to. Why Does White Zno (S) Becomes Yellow Upon Heating.
From www.doubtnut.com
ZnO is white cold and yellow when heated, it is due to the development Why Does White Zno (S) Becomes Yellow Upon Heating The excess zn ions thus formed move to the interstitial sites and electron in the. On heating zno, it loses oxygen and electrons, as shown below: When zinc oxide is heated strongly above ${800^ \circ }{\text{c}}$ it turns yellow. Why does white zno(s) become yellow upon heating? After cooling down, the colour of zinc oxide changes back to. Why does. Why Does White Zno (S) Becomes Yellow Upon Heating.
From www.doubtnut.com
[Punjabi] Why does white ZnO(s) yellow upon heating? Why Does White Zno (S) Becomes Yellow Upon Heating Why does white zno(s) become yellow upon heating? On heating, zno gives , electrons and colour. Why does white zn0(s) becomes yellow upon heating ? Zinc oxide, white in colour at room temperature, acquires yellow colour on heating due to: After cooling down, the colour of zinc oxide changes back to. In $\ce{zno}$, $\ce{zn}$ is present in the 2+ oxidation. Why Does White Zno (S) Becomes Yellow Upon Heating.
From www.youtube.com
How Does Zinc Oxide "UnBurn" Itself? YouTube Why Does White Zno (S) Becomes Yellow Upon Heating Zn 2+ ions and electrons move to interstitial sites and f. The excess zn ions thus formed move to the interstitial sites and electron in the. When zinc oxide is heated strongly above ${800^ \circ }{\text{c}}$ it turns yellow. The zno crystal becomes yellow oh heating because of the metal excess defect which is caused due to the presence of. Why Does White Zno (S) Becomes Yellow Upon Heating.
From www.doubtnut.com
[Punjabi] Why does ZnO appear golden yellow at high temperature? Expla Why Does White Zno (S) Becomes Yellow Upon Heating When zinc oxide is heated strongly above ${800^ \circ }{\text{c}}$ it turns yellow. Why does white zn0(s) becomes yellow upon heating ? Zn 2+ ions and electrons move to interstitial sites and f. Why does white zno(s) become yellow upon heating? On heating zno, it loses oxygen and electrons, as shown below: In $\ce{zno}$, $\ce{zn}$ is present in the 2+. Why Does White Zno (S) Becomes Yellow Upon Heating.
From www.studypool.com
SOLUTION Why does zinc oxide which is white in colour yellow Why Does White Zno (S) Becomes Yellow Upon Heating The excess zn ions thus formed move to the interstitial sites and electron in the. When zinc oxide is heated strongly above ${800^ \circ }{\text{c}}$ it turns yellow. Why does white zn0(s) becomes yellow upon heating ? On heating, zno gives , electrons and colour. On heating zno, it loses oxygen and electrons, as shown below: Why does white zno(s). Why Does White Zno (S) Becomes Yellow Upon Heating.
From www.researchgate.net
Schematic of the synthesis process for the white light R,ZnOZIF8WL Why Does White Zno (S) Becomes Yellow Upon Heating After cooling down, the colour of zinc oxide changes back to. On heating zno, it loses oxygen and electrons, as shown below: The zno crystal becomes yellow oh heating because of the metal excess defect which is caused due to the presence of extra cations at. When zinc oxide is heated strongly above ${800^ \circ }{\text{c}}$ it turns yellow. Zn. Why Does White Zno (S) Becomes Yellow Upon Heating.
From www.slideshare.net
Action of heat on chemical compound e book Why Does White Zno (S) Becomes Yellow Upon Heating The excess zn ions thus formed move to the interstitial sites and electron in the. On heating zno loses oxygen according to the following reaction. The zno crystal becomes yellow oh heating because of the metal excess defect which is caused due to the presence of extra cations at. On heating zno, it loses oxygen and electrons, as shown below:. Why Does White Zno (S) Becomes Yellow Upon Heating.
From chemistry-europe.onlinelibrary.wiley.com
Surfactant‐Mediated Electrochemical Precipitation of ZnO Nanospheres Why Does White Zno (S) Becomes Yellow Upon Heating After cooling down, the colour of zinc oxide changes back to. On heating zno, it loses oxygen and electrons, as shown below: The zno crystal becomes yellow oh heating because of the metal excess defect which is caused due to the presence of extra cations at. On heating zno loses oxygen according to the following reaction. Why does white zn0(s). Why Does White Zno (S) Becomes Yellow Upon Heating.
From www.youtube.com
`ZnO` is white cold and yellow when heated, it is due to the Why Does White Zno (S) Becomes Yellow Upon Heating The zno crystal becomes yellow oh heating because of the metal excess defect which is caused due to the presence of extra cations at. Zinc oxide, white in colour at room temperature, acquires yellow colour on heating due to: Zn 2+ ions and electrons move to interstitial sites and f. Why does white zno(s) become yellow upon heating? Why does. Why Does White Zno (S) Becomes Yellow Upon Heating.
From slideplayer.com
Topical Agents. ppt download Why Does White Zno (S) Becomes Yellow Upon Heating Why does white zn0(s) becomes yellow upon heating ? The excess zn ions thus formed move to the interstitial sites and electron in the. On heating zno loses oxygen according to the following reaction. The zno crystal becomes yellow oh heating because of the metal excess defect which is caused due to the presence of extra cations at. Why does. Why Does White Zno (S) Becomes Yellow Upon Heating.