What Is A Boiling Point Of Sand . The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid [1][2] and the liquid. 100 rows what is a boiling point? The boiling point of water is 100 °c at normal atmospheric pressure, at sea level. The normal boiling point or the atmospheric boiling point is the boiling point at 1 atmosphere of pressure or sea level. The simple science behind this 'boiling' sand. Mixtures melt and boil over a range of temperatures. At higher altitudes, the air pressure is lower, and water boils. The boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal to the surrounding. Pure substances have specific melting and boiling points. The standard boiling point, as defined by the iupac in 1982, is.
from www.vedantu.com
100 rows what is a boiling point? Mixtures melt and boil over a range of temperatures. The boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal to the surrounding. The normal boiling point or the atmospheric boiling point is the boiling point at 1 atmosphere of pressure or sea level. Pure substances have specific melting and boiling points. The standard boiling point, as defined by the iupac in 1982, is. The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid [1][2] and the liquid. The simple science behind this 'boiling' sand. The boiling point of water is 100 °c at normal atmospheric pressure, at sea level. At higher altitudes, the air pressure is lower, and water boils.
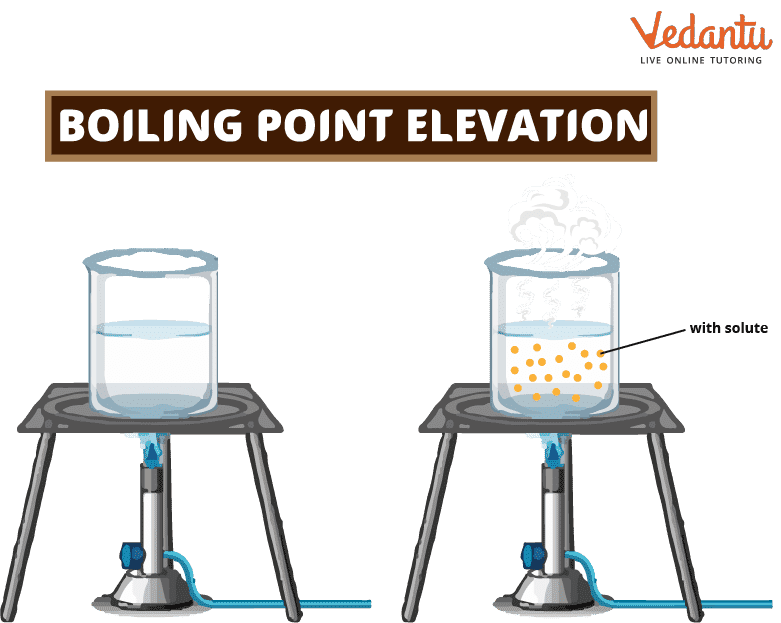
Boiling Point Elevation Learn Important Terms and Concepts
What Is A Boiling Point Of Sand The normal boiling point or the atmospheric boiling point is the boiling point at 1 atmosphere of pressure or sea level. The simple science behind this 'boiling' sand. The normal boiling point or the atmospheric boiling point is the boiling point at 1 atmosphere of pressure or sea level. The boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal to the surrounding. The standard boiling point, as defined by the iupac in 1982, is. The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid [1][2] and the liquid. Mixtures melt and boil over a range of temperatures. At higher altitudes, the air pressure is lower, and water boils. 100 rows what is a boiling point? The boiling point of water is 100 °c at normal atmospheric pressure, at sea level. Pure substances have specific melting and boiling points.
From seismicsolutionsllc.com
What is Liquefaction? Seismic Solutions LLC What Is A Boiling Point Of Sand The simple science behind this 'boiling' sand. Mixtures melt and boil over a range of temperatures. Pure substances have specific melting and boiling points. The boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal to the surrounding. The standard boiling point, as defined by the iupac in 1982, is. The boiling. What Is A Boiling Point Of Sand.
From www.wwltv.com
What is a sand boil? What Is A Boiling Point Of Sand The simple science behind this 'boiling' sand. 100 rows what is a boiling point? The normal boiling point or the atmospheric boiling point is the boiling point at 1 atmosphere of pressure or sea level. Pure substances have specific melting and boiling points. The boiling point of a substance is the temperature at which the vapor pressure of the liquid. What Is A Boiling Point Of Sand.
From klaubgmaz.blob.core.windows.net
What Is Boiling Point In Science at Lan Johnson blog What Is A Boiling Point Of Sand The simple science behind this 'boiling' sand. The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid [1][2] and the liquid. The standard boiling point, as defined by the iupac in 1982, is. The boiling point of a substance is the temperature at which the vapor pressure. What Is A Boiling Point Of Sand.
From www.slideserve.com
PPT Properties and Changes in Matter PowerPoint Presentation, free What Is A Boiling Point Of Sand The boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal to the surrounding. The normal boiling point or the atmospheric boiling point is the boiling point at 1 atmosphere of pressure or sea level. The boiling point of water is 100 °c at normal atmospheric pressure, at sea level. The boiling. What Is A Boiling Point Of Sand.
From apollo.lsc.vsc.edu
Saturation Vapor Pressure and the Boiling Point What Is A Boiling Point Of Sand The boiling point of water is 100 °c at normal atmospheric pressure, at sea level. At higher altitudes, the air pressure is lower, and water boils. The boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal to the surrounding. The simple science behind this 'boiling' sand. The boiling point of a. What Is A Boiling Point Of Sand.
From mavink.com
Melting And Boiling Point Chart What Is A Boiling Point Of Sand At higher altitudes, the air pressure is lower, and water boils. Mixtures melt and boil over a range of temperatures. The standard boiling point, as defined by the iupac in 1982, is. Pure substances have specific melting and boiling points. 100 rows what is a boiling point? The normal boiling point or the atmospheric boiling point is the boiling point. What Is A Boiling Point Of Sand.
From www.chemistrysteps.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps What Is A Boiling Point Of Sand The normal boiling point or the atmospheric boiling point is the boiling point at 1 atmosphere of pressure or sea level. The simple science behind this 'boiling' sand. The boiling point of water is 100 °c at normal atmospheric pressure, at sea level. Pure substances have specific melting and boiling points. The standard boiling point, as defined by the iupac. What Is A Boiling Point Of Sand.
From engineeringstuff.co.in
What is Boiling point Engineeringstuff What Is A Boiling Point Of Sand The boiling point of water is 100 °c at normal atmospheric pressure, at sea level. The simple science behind this 'boiling' sand. 100 rows what is a boiling point? The boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal to the surrounding. At higher altitudes, the air pressure is lower, and. What Is A Boiling Point Of Sand.
From www.globalperspectives.info
Boiling Point photos trend of February What Is A Boiling Point Of Sand The boiling point of water is 100 °c at normal atmospheric pressure, at sea level. Mixtures melt and boil over a range of temperatures. The normal boiling point or the atmospheric boiling point is the boiling point at 1 atmosphere of pressure or sea level. The standard boiling point, as defined by the iupac in 1982, is. The boiling point. What Is A Boiling Point Of Sand.
From www.researchgate.net
A representative true boiling point curve of crude oil. Download What Is A Boiling Point Of Sand At higher altitudes, the air pressure is lower, and water boils. The boiling point of water is 100 °c at normal atmospheric pressure, at sea level. The boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal to the surrounding. Mixtures melt and boil over a range of temperatures. The standard boiling. What Is A Boiling Point Of Sand.
From www.youtube.com
What is Boiling Point Concept of Boiling Point Boiling Point What Is A Boiling Point Of Sand Pure substances have specific melting and boiling points. 100 rows what is a boiling point? The simple science behind this 'boiling' sand. The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid [1][2] and the liquid. The boiling point of water is 100 °c at normal atmospheric. What Is A Boiling Point Of Sand.
From www.worksheetsplanet.com
What is Boiling Point What Is A Boiling Point Of Sand The normal boiling point or the atmospheric boiling point is the boiling point at 1 atmosphere of pressure or sea level. At higher altitudes, the air pressure is lower, and water boils. The standard boiling point, as defined by the iupac in 1982, is. 100 rows what is a boiling point? The boiling point of a substance is the temperature. What Is A Boiling Point Of Sand.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps What Is A Boiling Point Of Sand Mixtures melt and boil over a range of temperatures. The normal boiling point or the atmospheric boiling point is the boiling point at 1 atmosphere of pressure or sea level. The simple science behind this 'boiling' sand. The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid. What Is A Boiling Point Of Sand.
From www.vedantu.com
Boiling Point Elevation Learn Important Terms and Concepts What Is A Boiling Point Of Sand Pure substances have specific melting and boiling points. Mixtures melt and boil over a range of temperatures. The boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal to the surrounding. The normal boiling point or the atmospheric boiling point is the boiling point at 1 atmosphere of pressure or sea level.. What Is A Boiling Point Of Sand.
From klahkiyli.blob.core.windows.net
What Is Boiling Point Of Salt Water at Tracy Mendoza blog What Is A Boiling Point Of Sand The boiling point of water is 100 °c at normal atmospheric pressure, at sea level. The normal boiling point or the atmospheric boiling point is the boiling point at 1 atmosphere of pressure or sea level. The simple science behind this 'boiling' sand. The boiling point of a substance is the temperature at which the vapor pressure of a liquid. What Is A Boiling Point Of Sand.
From studiousguy.com
Boiling Point Examples in Everyday Life StudiousGuy What Is A Boiling Point Of Sand The simple science behind this 'boiling' sand. The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid [1][2] and the liquid. Mixtures melt and boil over a range of temperatures. The boiling point of water is 100 °c at normal atmospheric pressure, at sea level. At higher. What Is A Boiling Point Of Sand.
From www.learnatnoon.com
The boiling point of water and alcohol explained Noon Academy What Is A Boiling Point Of Sand Mixtures melt and boil over a range of temperatures. The boiling point of water is 100 °c at normal atmospheric pressure, at sea level. The simple science behind this 'boiling' sand. The normal boiling point or the atmospheric boiling point is the boiling point at 1 atmosphere of pressure or sea level. The standard boiling point, as defined by the. What Is A Boiling Point Of Sand.
From www.slideserve.com
PPT boiling point PowerPoint Presentation, free download ID2402961 What Is A Boiling Point Of Sand Mixtures melt and boil over a range of temperatures. The standard boiling point, as defined by the iupac in 1982, is. The simple science behind this 'boiling' sand. The boiling point of water is 100 °c at normal atmospheric pressure, at sea level. The normal boiling point or the atmospheric boiling point is the boiling point at 1 atmosphere of. What Is A Boiling Point Of Sand.
From chemistryskills.com
Definition and Explanation of Boiling Point Chemistry Skills What Is A Boiling Point Of Sand The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid [1][2] and the liquid. The normal boiling point or the atmospheric boiling point is the boiling point at 1 atmosphere of pressure or sea level. The simple science behind this 'boiling' sand. Mixtures melt and boil over. What Is A Boiling Point Of Sand.
From www.sliderbase.com
Bulk Properties of Water Presentation Chemistry What Is A Boiling Point Of Sand The boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal to the surrounding. 100 rows what is a boiling point? Pure substances have specific melting and boiling points. The normal boiling point or the atmospheric boiling point is the boiling point at 1 atmosphere of pressure or sea level. The simple. What Is A Boiling Point Of Sand.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? What Is A Boiling Point Of Sand The standard boiling point, as defined by the iupac in 1982, is. The boiling point of water is 100 °c at normal atmospheric pressure, at sea level. The normal boiling point or the atmospheric boiling point is the boiling point at 1 atmosphere of pressure or sea level. 100 rows what is a boiling point? The boiling point of a. What Is A Boiling Point Of Sand.
From www.slideserve.com
PPT Chapter Introduction Lesson 1 Physical Properties Lesson 2 What Is A Boiling Point Of Sand The simple science behind this 'boiling' sand. At higher altitudes, the air pressure is lower, and water boils. The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid [1][2] and the liquid. The normal boiling point or the atmospheric boiling point is the boiling point at 1. What Is A Boiling Point Of Sand.
From amootiranian.com
Sulfur Melting Point & Boiling Point What Is A Boiling Point Of Sand Pure substances have specific melting and boiling points. At higher altitudes, the air pressure is lower, and water boils. The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid [1][2] and the liquid. 100 rows what is a boiling point? The simple science behind this 'boiling' sand.. What Is A Boiling Point Of Sand.
From www.slideserve.com
PPT Boiling Point Notes PowerPoint Presentation, free download ID What Is A Boiling Point Of Sand 100 rows what is a boiling point? The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid [1][2] and the liquid. Pure substances have specific melting and boiling points. The boiling point of water is 100 °c at normal atmospheric pressure, at sea level. The simple science. What Is A Boiling Point Of Sand.
From www.physicsfox.org
Melting & Boiling • Matter • Physics Fox What Is A Boiling Point Of Sand The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid [1][2] and the liquid. The standard boiling point, as defined by the iupac in 1982, is. The normal boiling point or the atmospheric boiling point is the boiling point at 1 atmosphere of pressure or sea level.. What Is A Boiling Point Of Sand.
From www.youtube.com
Boiling Sand YouTube What Is A Boiling Point Of Sand At higher altitudes, the air pressure is lower, and water boils. Pure substances have specific melting and boiling points. The standard boiling point, as defined by the iupac in 1982, is. 100 rows what is a boiling point? The boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal to the surrounding.. What Is A Boiling Point Of Sand.
From www.youtube.com
What is boiling point what is the boiling point Factors affecting What Is A Boiling Point Of Sand 100 rows what is a boiling point? The normal boiling point or the atmospheric boiling point is the boiling point at 1 atmosphere of pressure or sea level. Pure substances have specific melting and boiling points. Mixtures melt and boil over a range of temperatures. The boiling point of a substance is the temperature at which the vapor pressure of. What Is A Boiling Point Of Sand.
From facts.net
20 Fascinating Facts About Boiling Point Elevation What Is A Boiling Point Of Sand The simple science behind this 'boiling' sand. The normal boiling point or the atmospheric boiling point is the boiling point at 1 atmosphere of pressure or sea level. Pure substances have specific melting and boiling points. 100 rows what is a boiling point? At higher altitudes, the air pressure is lower, and water boils. The boiling point of a substance. What Is A Boiling Point Of Sand.
From sciencenotes.org
Boiling Point Definition, Temperature, and Examples What Is A Boiling Point Of Sand 100 rows what is a boiling point? The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid [1][2] and the liquid. The boiling point of water is 100 °c at normal atmospheric pressure, at sea level. Pure substances have specific melting and boiling points. The normal boiling. What Is A Boiling Point Of Sand.
From chemistnotes.com
Boiling Point Definition, Boiling point as physical properties What Is A Boiling Point Of Sand The boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal to the surrounding. The normal boiling point or the atmospheric boiling point is the boiling point at 1 atmosphere of pressure or sea level. At higher altitudes, the air pressure is lower, and water boils. The boiling point of a substance. What Is A Boiling Point Of Sand.
From www.chegg.com
Solved Consider The Upward Flow Of Water Through A Layer What Is A Boiling Point Of Sand Mixtures melt and boil over a range of temperatures. The normal boiling point or the atmospheric boiling point is the boiling point at 1 atmosphere of pressure or sea level. 100 rows what is a boiling point? The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid. What Is A Boiling Point Of Sand.
From sciencenotes.org
Boiling Point Definition, Temperature, and Examples What Is A Boiling Point Of Sand The normal boiling point or the atmospheric boiling point is the boiling point at 1 atmosphere of pressure or sea level. The simple science behind this 'boiling' sand. Pure substances have specific melting and boiling points. At higher altitudes, the air pressure is lower, and water boils. The boiling point of a substance is the temperature at which the vapor. What Is A Boiling Point Of Sand.
From www.chemistrysteps.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps What Is A Boiling Point Of Sand The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid [1][2] and the liquid. The standard boiling point, as defined by the iupac in 1982, is. At higher altitudes, the air pressure is lower, and water boils. The simple science behind this 'boiling' sand. 100 rows what. What Is A Boiling Point Of Sand.
From ceghryvh.blob.core.windows.net
What Is Boiling Point Sea Level at Edwin Acosta blog What Is A Boiling Point Of Sand The boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal to the surrounding. Mixtures melt and boil over a range of temperatures. 100 rows what is a boiling point? The normal boiling point or the atmospheric boiling point is the boiling point at 1 atmosphere of pressure or sea level. The. What Is A Boiling Point Of Sand.
From www.youtube.com
Binary Boiling Point Diagram of a LiquidLiquid Mixture YouTube What Is A Boiling Point Of Sand At higher altitudes, the air pressure is lower, and water boils. Mixtures melt and boil over a range of temperatures. The simple science behind this 'boiling' sand. Pure substances have specific melting and boiling points. 100 rows what is a boiling point? The normal boiling point or the atmospheric boiling point is the boiling point at 1 atmosphere of pressure. What Is A Boiling Point Of Sand.