Titration Of Phosphoric Acid With Naoh Lab Report . titration of the phosphoric acid h 3 po 4 is an interesting case. • determine the first dissociation. titration of phosphoric acid with your volumetric pipet, measure 25.00 ml of 0.06 m (nominal) phosphoric acid into a 100 ml. • experience the titration of a triprotic acid. to determine the amount of acid in an unknown sample, you will need to add a known amount of base until the acid and. Although often listed together with strong mineral acids (hydrochloric, nitric. in this experiment, we will determine two of the three pka values for the triprotic acid, phosphoric acid, using acid. It is the major acid that. • learn proper technique in pipette and buret usage. in this experiment you will titrate h3po4 with a dilute solution of naoh.
from general.chemistrysteps.com
• determine the first dissociation. • learn proper technique in pipette and buret usage. titration of phosphoric acid with your volumetric pipet, measure 25.00 ml of 0.06 m (nominal) phosphoric acid into a 100 ml. in this experiment you will titrate h3po4 with a dilute solution of naoh. Although often listed together with strong mineral acids (hydrochloric, nitric. to determine the amount of acid in an unknown sample, you will need to add a known amount of base until the acid and. • experience the titration of a triprotic acid. It is the major acid that. titration of the phosphoric acid h 3 po 4 is an interesting case. in this experiment, we will determine two of the three pka values for the triprotic acid, phosphoric acid, using acid.
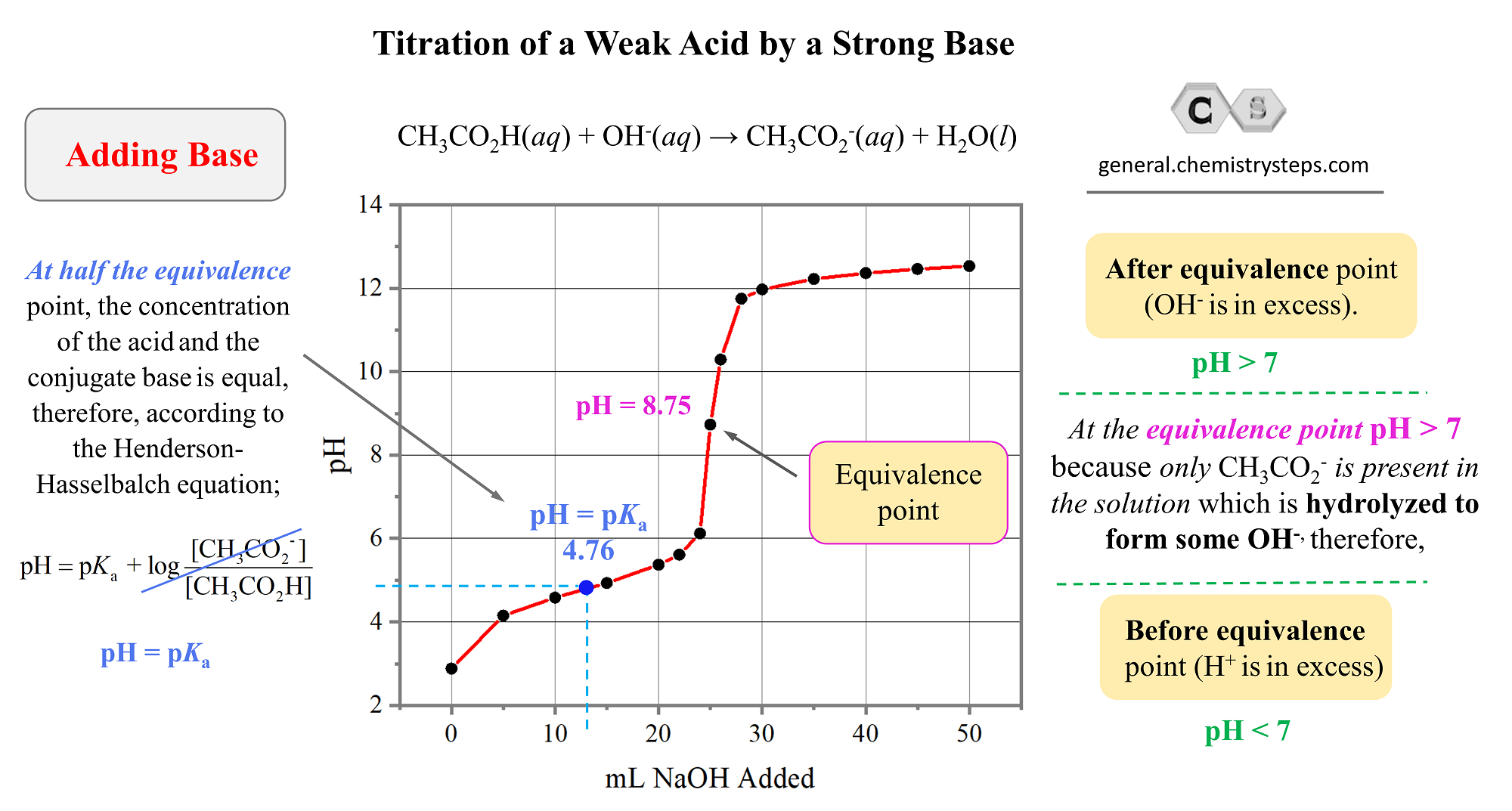
Titration of a Weak Acid by a Strong Base Chemistry Steps
Titration Of Phosphoric Acid With Naoh Lab Report Although often listed together with strong mineral acids (hydrochloric, nitric. in this experiment you will titrate h3po4 with a dilute solution of naoh. to determine the amount of acid in an unknown sample, you will need to add a known amount of base until the acid and. • determine the first dissociation. • learn proper technique in pipette and buret usage. Although often listed together with strong mineral acids (hydrochloric, nitric. in this experiment, we will determine two of the three pka values for the triprotic acid, phosphoric acid, using acid. It is the major acid that. titration of the phosphoric acid h 3 po 4 is an interesting case. titration of phosphoric acid with your volumetric pipet, measure 25.00 ml of 0.06 m (nominal) phosphoric acid into a 100 ml. • experience the titration of a triprotic acid.
From www.youtube.com
Titration of Phosphoric Acid and Acetic Acid with NaOH YouTube Titration Of Phosphoric Acid With Naoh Lab Report in this experiment, we will determine two of the three pka values for the triprotic acid, phosphoric acid, using acid. • experience the titration of a triprotic acid. Although often listed together with strong mineral acids (hydrochloric, nitric. titration of phosphoric acid with your volumetric pipet, measure 25.00 ml of 0.06 m (nominal) phosphoric acid into a. Titration Of Phosphoric Acid With Naoh Lab Report.
From aylinmeowstuart.blogspot.com
Acid Base Titration Experiment Report Titration Of Phosphoric Acid With Naoh Lab Report to determine the amount of acid in an unknown sample, you will need to add a known amount of base until the acid and. in this experiment you will titrate h3po4 with a dilute solution of naoh. It is the major acid that. • learn proper technique in pipette and buret usage. Although often listed together with strong. Titration Of Phosphoric Acid With Naoh Lab Report.
From general.chemistrysteps.com
Titration of a Weak Acid by a Strong Base Chemistry Steps Titration Of Phosphoric Acid With Naoh Lab Report • experience the titration of a triprotic acid. It is the major acid that. to determine the amount of acid in an unknown sample, you will need to add a known amount of base until the acid and. • determine the first dissociation. in this experiment you will titrate h3po4 with a dilute solution of naoh. . Titration Of Phosphoric Acid With Naoh Lab Report.
From mungfali.com
Acid Base Titration Lab Titration Of Phosphoric Acid With Naoh Lab Report • determine the first dissociation. in this experiment you will titrate h3po4 with a dilute solution of naoh. Although often listed together with strong mineral acids (hydrochloric, nitric. in this experiment, we will determine two of the three pka values for the triprotic acid, phosphoric acid, using acid. titration of phosphoric acid with your volumetric pipet, measure. Titration Of Phosphoric Acid With Naoh Lab Report.
From exoptndih.blob.core.windows.net
Acid Base Titration Lab Answers Hcl Naoh at Daniel Tilley blog Titration Of Phosphoric Acid With Naoh Lab Report to determine the amount of acid in an unknown sample, you will need to add a known amount of base until the acid and. titration of phosphoric acid with your volumetric pipet, measure 25.00 ml of 0.06 m (nominal) phosphoric acid into a 100 ml. • learn proper technique in pipette and buret usage. • experience the. Titration Of Phosphoric Acid With Naoh Lab Report.
From www.numerade.com
SOLVED Determine the pH at the equivalence point of titration of Titration Of Phosphoric Acid With Naoh Lab Report • determine the first dissociation. It is the major acid that. in this experiment, we will determine two of the three pka values for the triprotic acid, phosphoric acid, using acid. Although often listed together with strong mineral acids (hydrochloric, nitric. • learn proper technique in pipette and buret usage. to determine the amount of acid in an. Titration Of Phosphoric Acid With Naoh Lab Report.
From www.chegg.com
Solved Report sheet Exp. 8 Phosphoric Acid in Cola Lab Titration Of Phosphoric Acid With Naoh Lab Report to determine the amount of acid in an unknown sample, you will need to add a known amount of base until the acid and. in this experiment, we will determine two of the three pka values for the triprotic acid, phosphoric acid, using acid. It is the major acid that. titration of phosphoric acid with your volumetric. Titration Of Phosphoric Acid With Naoh Lab Report.
From exoptndih.blob.core.windows.net
Acid Base Titration Lab Answers Hcl Naoh at Daniel Tilley blog Titration Of Phosphoric Acid With Naoh Lab Report It is the major acid that. titration of phosphoric acid with your volumetric pipet, measure 25.00 ml of 0.06 m (nominal) phosphoric acid into a 100 ml. in this experiment, we will determine two of the three pka values for the triprotic acid, phosphoric acid, using acid. in this experiment you will titrate h3po4 with a dilute. Titration Of Phosphoric Acid With Naoh Lab Report.
From www.studocu.com
Lab report Titration of HCl with NaOH Prelab Title Titration of Titration Of Phosphoric Acid With Naoh Lab Report • learn proper technique in pipette and buret usage. in this experiment you will titrate h3po4 with a dilute solution of naoh. titration of the phosphoric acid h 3 po 4 is an interesting case. titration of phosphoric acid with your volumetric pipet, measure 25.00 ml of 0.06 m (nominal) phosphoric acid into a 100 ml. . Titration Of Phosphoric Acid With Naoh Lab Report.
From www.studocu.com
Titration Lab Report Acid Base Titration Lab Report Table 3 NaOH Titration Of Phosphoric Acid With Naoh Lab Report • experience the titration of a triprotic acid. titration of phosphoric acid with your volumetric pipet, measure 25.00 ml of 0.06 m (nominal) phosphoric acid into a 100 ml. titration of the phosphoric acid h 3 po 4 is an interesting case. in this experiment, we will determine two of the three pka values for the. Titration Of Phosphoric Acid With Naoh Lab Report.
From www.vrogue.co
Solution Chemistry Acid Base Titration Studypool vrogue.co Titration Of Phosphoric Acid With Naoh Lab Report in this experiment you will titrate h3po4 with a dilute solution of naoh. titration of phosphoric acid with your volumetric pipet, measure 25.00 ml of 0.06 m (nominal) phosphoric acid into a 100 ml. titration of the phosphoric acid h 3 po 4 is an interesting case. in this experiment, we will determine two of the. Titration Of Phosphoric Acid With Naoh Lab Report.
From www.scribd.com
Phosphoric Acid Titration PDF Titration Chemistry Titration Of Phosphoric Acid With Naoh Lab Report titration of phosphoric acid with your volumetric pipet, measure 25.00 ml of 0.06 m (nominal) phosphoric acid into a 100 ml. • experience the titration of a triprotic acid. Although often listed together with strong mineral acids (hydrochloric, nitric. It is the major acid that. • learn proper technique in pipette and buret usage. in this experiment,. Titration Of Phosphoric Acid With Naoh Lab Report.
From www.youtube.com
REPRESENTING THE TITRATION CURVE OF PHOSPHORIC ACID YouTube Titration Of Phosphoric Acid With Naoh Lab Report to determine the amount of acid in an unknown sample, you will need to add a known amount of base until the acid and. • experience the titration of a triprotic acid. • determine the first dissociation. in this experiment you will titrate h3po4 with a dilute solution of naoh. • learn proper technique in pipette and. Titration Of Phosphoric Acid With Naoh Lab Report.
From dokumen.tips
(PDF) Phosphoric Acid Titration Ohlone College Acid.pdf · 1 Titration Of Phosphoric Acid With Naoh Lab Report titration of phosphoric acid with your volumetric pipet, measure 25.00 ml of 0.06 m (nominal) phosphoric acid into a 100 ml. It is the major acid that. to determine the amount of acid in an unknown sample, you will need to add a known amount of base until the acid and. • learn proper technique in pipette and. Titration Of Phosphoric Acid With Naoh Lab Report.
From www.numerade.com
SOLVED Table 2. Titration of phosphoric acid in a soft drink with Titration Of Phosphoric Acid With Naoh Lab Report Although often listed together with strong mineral acids (hydrochloric, nitric. titration of the phosphoric acid h 3 po 4 is an interesting case. to determine the amount of acid in an unknown sample, you will need to add a known amount of base until the acid and. in this experiment you will titrate h3po4 with a dilute. Titration Of Phosphoric Acid With Naoh Lab Report.
From www.youtube.com
AP Lecture Phosphoric Acid Titration YouTube Titration Of Phosphoric Acid With Naoh Lab Report Although often listed together with strong mineral acids (hydrochloric, nitric. in this experiment, we will determine two of the three pka values for the triprotic acid, phosphoric acid, using acid. titration of the phosphoric acid h 3 po 4 is an interesting case. in this experiment you will titrate h3po4 with a dilute solution of naoh. . Titration Of Phosphoric Acid With Naoh Lab Report.
From www.numerade.com
SOLVED Part III Titration Standardization of NaOH solution (finding Titration Of Phosphoric Acid With Naoh Lab Report Although often listed together with strong mineral acids (hydrochloric, nitric. in this experiment, we will determine two of the three pka values for the triprotic acid, phosphoric acid, using acid. • determine the first dissociation. It is the major acid that. in this experiment you will titrate h3po4 with a dilute solution of naoh. titration of the. Titration Of Phosphoric Acid With Naoh Lab Report.
From www.numerade.com
SOLVED 14.Write the equilibrium constant expression for the Titration Of Phosphoric Acid With Naoh Lab Report Although often listed together with strong mineral acids (hydrochloric, nitric. It is the major acid that. titration of the phosphoric acid h 3 po 4 is an interesting case. • learn proper technique in pipette and buret usage. in this experiment, we will determine two of the three pka values for the triprotic acid, phosphoric acid, using acid.. Titration Of Phosphoric Acid With Naoh Lab Report.
From www.numerade.com
SOLVED Post LAB AcidBase Titration Name Dale Write the balanced Titration Of Phosphoric Acid With Naoh Lab Report to determine the amount of acid in an unknown sample, you will need to add a known amount of base until the acid and. titration of the phosphoric acid h 3 po 4 is an interesting case. Although often listed together with strong mineral acids (hydrochloric, nitric. in this experiment, we will determine two of the three. Titration Of Phosphoric Acid With Naoh Lab Report.
From www.studypool.com
SOLUTION Experiment 7 Report titration of phosphoric acid and acetic Titration Of Phosphoric Acid With Naoh Lab Report Although often listed together with strong mineral acids (hydrochloric, nitric. It is the major acid that. • learn proper technique in pipette and buret usage. • experience the titration of a triprotic acid. in this experiment, we will determine two of the three pka values for the triprotic acid, phosphoric acid, using acid. to determine the amount. Titration Of Phosphoric Acid With Naoh Lab Report.
From www.scribd.com
Titration Of Phosphoric Acid And A Cola Beverage With Sodium Hydroxide Titration Of Phosphoric Acid With Naoh Lab Report in this experiment you will titrate h3po4 with a dilute solution of naoh. to determine the amount of acid in an unknown sample, you will need to add a known amount of base until the acid and. • experience the titration of a triprotic acid. titration of the phosphoric acid h 3 po 4 is an. Titration Of Phosphoric Acid With Naoh Lab Report.
From www.numerade.com
SOLVED Post LAB AcidBase Titration Name Dale Write the balanced Titration Of Phosphoric Acid With Naoh Lab Report titration of the phosphoric acid h 3 po 4 is an interesting case. Although often listed together with strong mineral acids (hydrochloric, nitric. titration of phosphoric acid with your volumetric pipet, measure 25.00 ml of 0.06 m (nominal) phosphoric acid into a 100 ml. • learn proper technique in pipette and buret usage. in this experiment you. Titration Of Phosphoric Acid With Naoh Lab Report.
From www.showme.com
ShowMe Titration Of Phosphoric acid With Sodium Hydroxide Titration Of Phosphoric Acid With Naoh Lab Report in this experiment you will titrate h3po4 with a dilute solution of naoh. • determine the first dissociation. It is the major acid that. • learn proper technique in pipette and buret usage. titration of the phosphoric acid h 3 po 4 is an interesting case. Although often listed together with strong mineral acids (hydrochloric, nitric. in. Titration Of Phosphoric Acid With Naoh Lab Report.
From www.chegg.com
Solved In a titration, a 25.0 cm3 sample of phosphoric acid Titration Of Phosphoric Acid With Naoh Lab Report It is the major acid that. in this experiment, we will determine two of the three pka values for the triprotic acid, phosphoric acid, using acid. titration of the phosphoric acid h 3 po 4 is an interesting case. • experience the titration of a triprotic acid. • determine the first dissociation. to determine the amount. Titration Of Phosphoric Acid With Naoh Lab Report.
From www.chegg.com
Solved REPORT SHEET EXPERIMENT Analysis of Phosphoric Acid Titration Of Phosphoric Acid With Naoh Lab Report • learn proper technique in pipette and buret usage. titration of the phosphoric acid h 3 po 4 is an interesting case. to determine the amount of acid in an unknown sample, you will need to add a known amount of base until the acid and. Although often listed together with strong mineral acids (hydrochloric, nitric. It is. Titration Of Phosphoric Acid With Naoh Lab Report.
From www.numerade.com
SOLVED Experiment 19 PostLaboratory Questions An impure which Titration Of Phosphoric Acid With Naoh Lab Report It is the major acid that. Although often listed together with strong mineral acids (hydrochloric, nitric. in this experiment, we will determine two of the three pka values for the triprotic acid, phosphoric acid, using acid. to determine the amount of acid in an unknown sample, you will need to add a known amount of base until the. Titration Of Phosphoric Acid With Naoh Lab Report.
From www.studypool.com
SOLUTION Conductometric titration II lab report Studypool Titration Of Phosphoric Acid With Naoh Lab Report • determine the first dissociation. • learn proper technique in pipette and buret usage. to determine the amount of acid in an unknown sample, you will need to add a known amount of base until the acid and. in this experiment you will titrate h3po4 with a dilute solution of naoh. in this experiment, we will determine. Titration Of Phosphoric Acid With Naoh Lab Report.
From www.studypool.com
SOLUTION Experiment 7 Report titration of phosphoric acid and acetic Titration Of Phosphoric Acid With Naoh Lab Report in this experiment, we will determine two of the three pka values for the triprotic acid, phosphoric acid, using acid. in this experiment you will titrate h3po4 with a dilute solution of naoh. • experience the titration of a triprotic acid. titration of phosphoric acid with your volumetric pipet, measure 25.00 ml of 0.06 m (nominal). Titration Of Phosphoric Acid With Naoh Lab Report.
From www.studypool.com
SOLUTION Experiment 7 Report titration of phosphoric acid and acetic Titration Of Phosphoric Acid With Naoh Lab Report • experience the titration of a triprotic acid. titration of phosphoric acid with your volumetric pipet, measure 25.00 ml of 0.06 m (nominal) phosphoric acid into a 100 ml. to determine the amount of acid in an unknown sample, you will need to add a known amount of base until the acid and. Although often listed together. Titration Of Phosphoric Acid With Naoh Lab Report.
From www.numerade.com
SOLVED Text EXPERIMENT 6 ACIDBASE TITRATIONS LABORATORY REPORT SHEET Titration Of Phosphoric Acid With Naoh Lab Report • learn proper technique in pipette and buret usage. in this experiment you will titrate h3po4 with a dilute solution of naoh. • determine the first dissociation. to determine the amount of acid in an unknown sample, you will need to add a known amount of base until the acid and. • experience the titration of a. Titration Of Phosphoric Acid With Naoh Lab Report.
From asideload7.gitlab.io
Nice Khp Naoh Titration Calculations List Of All Physics Formulas Titration Of Phosphoric Acid With Naoh Lab Report It is the major acid that. • experience the titration of a triprotic acid. in this experiment, we will determine two of the three pka values for the triprotic acid, phosphoric acid, using acid. • determine the first dissociation. to determine the amount of acid in an unknown sample, you will need to add a known amount. Titration Of Phosphoric Acid With Naoh Lab Report.
From www.studypool.com
SOLUTION Experiment 7 Report titration of phosphoric acid and acetic Titration Of Phosphoric Acid With Naoh Lab Report • experience the titration of a triprotic acid. titration of phosphoric acid with your volumetric pipet, measure 25.00 ml of 0.06 m (nominal) phosphoric acid into a 100 ml. It is the major acid that. in this experiment you will titrate h3po4 with a dilute solution of naoh. titration of the phosphoric acid h 3 po. Titration Of Phosphoric Acid With Naoh Lab Report.
From www.studocu.com
Lab6 lab report CHM 106 Potentiometric Titration of Phosphoric Acid Titration Of Phosphoric Acid With Naoh Lab Report Although often listed together with strong mineral acids (hydrochloric, nitric. titration of phosphoric acid with your volumetric pipet, measure 25.00 ml of 0.06 m (nominal) phosphoric acid into a 100 ml. It is the major acid that. • determine the first dissociation. in this experiment you will titrate h3po4 with a dilute solution of naoh. to determine. Titration Of Phosphoric Acid With Naoh Lab Report.
From asideload7.gitlab.io
Nice Khp Naoh Titration Calculations List Of All Physics Formulas Titration Of Phosphoric Acid With Naoh Lab Report in this experiment, we will determine two of the three pka values for the triprotic acid, phosphoric acid, using acid. • learn proper technique in pipette and buret usage. titration of phosphoric acid with your volumetric pipet, measure 25.00 ml of 0.06 m (nominal) phosphoric acid into a 100 ml. • experience the titration of a triprotic. Titration Of Phosphoric Acid With Naoh Lab Report.
From www.numerade.com
SOLVED Part € Titration of Phosphoric Acid Obtain the pH of Titration Of Phosphoric Acid With Naoh Lab Report It is the major acid that. • experience the titration of a triprotic acid. in this experiment you will titrate h3po4 with a dilute solution of naoh. to determine the amount of acid in an unknown sample, you will need to add a known amount of base until the acid and. • determine the first dissociation. . Titration Of Phosphoric Acid With Naoh Lab Report.