What Is An Electron Shielding Effect . electrons in an atom can shield each other from the pull of the nucleus. The orbital (n) and subshell (ml) define how close an electron can. the balance between attractive and repulsive forces results in shielding. This effect, called the shielding effect, describes the decrease in attraction. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because of the spherical shape of the \ (s\) orbital.
from www.slideserve.com
The orbital (n) and subshell (ml) define how close an electron can. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. electrons in an atom can shield each other from the pull of the nucleus. the balance between attractive and repulsive forces results in shielding. Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because of the spherical shape of the \ (s\) orbital. This effect, called the shielding effect, describes the decrease in attraction.
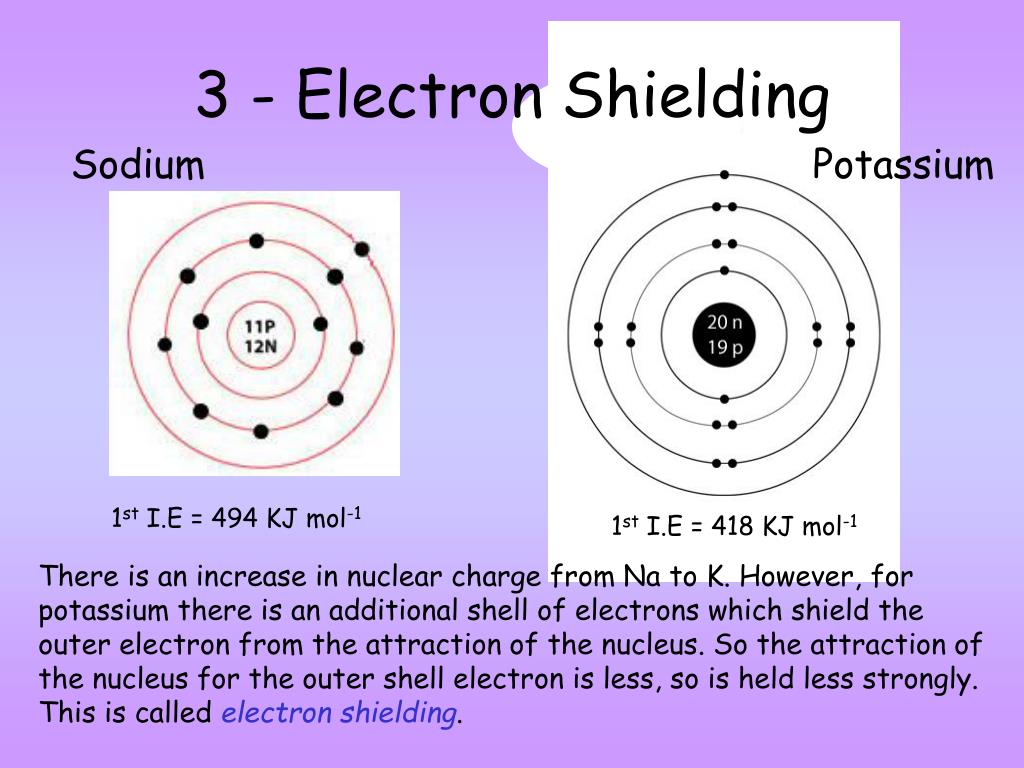
PPT Lesson objectives • Define first ionisation energy and successive
What Is An Electron Shielding Effect The orbital (n) and subshell (ml) define how close an electron can. This effect, called the shielding effect, describes the decrease in attraction. the balance between attractive and repulsive forces results in shielding. Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because of the spherical shape of the \ (s\) orbital. electrons in an atom can shield each other from the pull of the nucleus. The orbital (n) and subshell (ml) define how close an electron can. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use.
From www.youtube.com
SHIELDING/SCREENING EFFECT YouTube What Is An Electron Shielding Effect The orbital (n) and subshell (ml) define how close an electron can. electrons in an atom can shield each other from the pull of the nucleus. Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because of the spherical shape of the \ (s\) orbital. Effective nuclear charge, zeff, experienced by an. What Is An Electron Shielding Effect.
From www.youtube.com
Shielding Effect in the Periodic Table Chemistry YouTube What Is An Electron Shielding Effect the balance between attractive and repulsive forces results in shielding. electrons in an atom can shield each other from the pull of the nucleus. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. This effect, called the shielding effect, describes the decrease in. What Is An Electron Shielding Effect.
From www.worksheetsplanet.com
What Is An Electron What Is An Electron Shielding Effect Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because of the spherical shape of the \ (s\) orbital. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. The orbital (n) and subshell (ml) define how close an. What Is An Electron Shielding Effect.
From ar.inspiredpencil.com
Shielding Effect Electrons What Is An Electron Shielding Effect the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because of the spherical shape of the \ (s\) orbital. The orbital (n) and subshell (ml) define how close an. What Is An Electron Shielding Effect.
From chemisfast.blogspot.com
Lanthanide contractiondefinitioncausesconsequences in chemistry PG What Is An Electron Shielding Effect Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because of the spherical shape of the \ (s\) orbital. The orbital (n) and subshell (ml) define how close an electron can. the balance between attractive and repulsive forces results in shielding. This effect, called the shielding effect, describes the decrease in attraction.. What Is An Electron Shielding Effect.
From www.slideserve.com
PPT The Periodic Table and Physical Properties PowerPoint What Is An Electron Shielding Effect the balance between attractive and repulsive forces results in shielding. electrons in an atom can shield each other from the pull of the nucleus. This effect, called the shielding effect, describes the decrease in attraction. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the. What Is An Electron Shielding Effect.
From byjus.com
What is shielding and deshielding in NMR? Give an example? What Is An Electron Shielding Effect the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because of the spherical shape of the \ (s\) orbital. the balance between attractive and repulsive forces results in. What Is An Electron Shielding Effect.
From slidesharetrick.blogspot.com
What Is Electron Shielding slidesharetrick What Is An Electron Shielding Effect electrons in an atom can shield each other from the pull of the nucleus. Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because of the spherical shape of the \ (s\) orbital. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. This. What Is An Electron Shielding Effect.
From www.slideserve.com
PPT Periodic Table PowerPoint Presentation ID2834450 What Is An Electron Shielding Effect This effect, called the shielding effect, describes the decrease in attraction. electrons in an atom can shield each other from the pull of the nucleus. the balance between attractive and repulsive forces results in shielding. Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because of the spherical shape of the. What Is An Electron Shielding Effect.
From chemistnotes.com
Shielding Effect or Screening Effect Definition, Factors Affecting What Is An Electron Shielding Effect The orbital (n) and subshell (ml) define how close an electron can. This effect, called the shielding effect, describes the decrease in attraction. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. electrons in an atom can shield each other from the pull of the nucleus. Electrons in an \. What Is An Electron Shielding Effect.
From ar.inspiredpencil.com
Electron Shielding What Is An Electron Shielding Effect Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. This effect, called the shielding effect, describes the decrease in attraction. the balance between attractive and repulsive forces results in shielding. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an. What Is An Electron Shielding Effect.
From fyotqxgoy.blob.core.windows.net
What Is Electron Shielding Mean at Tobias Richter blog What Is An Electron Shielding Effect This effect, called the shielding effect, describes the decrease in attraction. Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because of the spherical shape of the \ (s\) orbital. electrons in an atom can shield each other from the pull of the nucleus. Effective nuclear charge, zeff, experienced by an electron. What Is An Electron Shielding Effect.
From ar.inspiredpencil.com
Shielding Effect Trend In Periodic Table What Is An Electron Shielding Effect the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. electrons in an atom can shield each other from the pull of the nucleus. The orbital (n) and subshell (ml) define how close an electron can. the balance between attractive and repulsive forces results. What Is An Electron Shielding Effect.
From www.showme.com
Electron shielding Science, Chemistry, Electrons, electron shielding What Is An Electron Shielding Effect electrons in an atom can shield each other from the pull of the nucleus. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. the balance. What Is An Electron Shielding Effect.
From simpleenglishchemistry.blogspot.com
Simple English Chemistry Atomic Size/Atomic Radius, Electronegativity What Is An Electron Shielding Effect the balance between attractive and repulsive forces results in shielding. electrons in an atom can shield each other from the pull of the nucleus. This effect, called the shielding effect, describes the decrease in attraction. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. Electrons in an \ (s\). What Is An Electron Shielding Effect.
From slidesharetrick.blogspot.com
What Is Electron Shielding slidesharetrick What Is An Electron Shielding Effect the balance between attractive and repulsive forces results in shielding. The orbital (n) and subshell (ml) define how close an electron can. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because of the. What Is An Electron Shielding Effect.
From www.slideserve.com
PPT Periodic Table Chapter PowerPoint Presentation, free download What Is An Electron Shielding Effect the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because of the spherical shape of the \ (s\) orbital. The orbital (n) and subshell (ml) define how close an. What Is An Electron Shielding Effect.
From fyogkgmgf.blob.core.windows.net
What Causes Shielding Effect at Don Bader blog What Is An Electron Shielding Effect electrons in an atom can shield each other from the pull of the nucleus. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because of the spherical shape of the \ (s\) orbital. The. What Is An Electron Shielding Effect.
From hxewxtchm.blob.core.windows.net
Why Is Electron Shielding Not A Factor When You at Veronica Gries blog What Is An Electron Shielding Effect This effect, called the shielding effect, describes the decrease in attraction. electrons in an atom can shield each other from the pull of the nucleus. Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because of the spherical shape of the \ (s\) orbital. the balance between attractive and repulsive forces. What Is An Electron Shielding Effect.
From www.youtube.com
Chapter 3 Shielding Effect and Atomic Size YouTube What Is An Electron Shielding Effect Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because of the spherical shape of the \ (s\) orbital. electrons in an atom can shield each other from the pull of the nucleus. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by. What Is An Electron Shielding Effect.
From slidesharetrick.blogspot.com
What Is Electron Shielding slidesharetrick What Is An Electron Shielding Effect the balance between attractive and repulsive forces results in shielding. This effect, called the shielding effect, describes the decrease in attraction. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same. What Is An Electron Shielding Effect.
From fyotqxgoy.blob.core.windows.net
What Is Electron Shielding Mean at Tobias Richter blog What Is An Electron Shielding Effect the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. the balance between attractive and repulsive forces results in shielding. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. Electrons in an \ (s\) orbital can. What Is An Electron Shielding Effect.
From chemisfast.blogspot.com
What is effect ?How does effect influence the What Is An Electron Shielding Effect Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because of the spherical shape of the \ (s\) orbital. electrons in an atom can shield each other from the pull of the nucleus. the balance between attractive and repulsive forces results in shielding. The orbital (n) and subshell (ml) define how. What Is An Electron Shielding Effect.
From ar.inspiredpencil.com
Shielding Effect Electrons What Is An Electron Shielding Effect Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because of the spherical shape of the \ (s\) orbital. The orbital (n) and subshell (ml) define how close an electron can. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows. What Is An Electron Shielding Effect.
From byjus.com
64. The order of screening effect of electrons of s p d and f orbital What Is An Electron Shielding Effect Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. This effect, called the shielding effect, describes the decrease in attraction. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. the balance between attractive and repulsive. What Is An Electron Shielding Effect.
From surfguppy.com
What is Electronegativity? What Is An Electron Shielding Effect electrons in an atom can shield each other from the pull of the nucleus. the balance between attractive and repulsive forces results in shielding. Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because of the spherical shape of the \ (s\) orbital. The orbital (n) and subshell (ml) define how. What Is An Electron Shielding Effect.
From www.slideserve.com
PPT Lesson objectives • Define first ionisation energy and successive What Is An Electron Shielding Effect electrons in an atom can shield each other from the pull of the nucleus. The orbital (n) and subshell (ml) define how close an electron can. the balance between attractive and repulsive forces results in shielding. This effect, called the shielding effect, describes the decrease in attraction. the concept of electron shielding, in which intervening electrons act. What Is An Electron Shielding Effect.
From scienceinfo.com
Shielding effect What Is An Electron Shielding Effect the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. This effect, called the shielding effect, describes the decrease in attraction. electrons in an atom can shield each other from the pull of the nucleus. Effective nuclear charge, zeff, experienced by an electron is less. What Is An Electron Shielding Effect.
From www.youtube.com
S3.1.3 Electron shielding and effective nuclear charge YouTube What Is An Electron Shielding Effect This effect, called the shielding effect, describes the decrease in attraction. Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because of the spherical shape of the \ (s\) orbital. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the. What Is An Electron Shielding Effect.
From www.slideserve.com
PPT Periodic Table PowerPoint Presentation, free download ID1951146 What Is An Electron Shielding Effect electrons in an atom can shield each other from the pull of the nucleus. the balance between attractive and repulsive forces results in shielding. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level. What Is An Electron Shielding Effect.
From socratic.org
How are shielding effect and atomic radius related? Socratic What Is An Electron Shielding Effect electrons in an atom can shield each other from the pull of the nucleus. This effect, called the shielding effect, describes the decrease in attraction. Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because of the spherical shape of the \ (s\) orbital. the balance between attractive and repulsive forces. What Is An Electron Shielding Effect.
From ecurrencythailand.com
What Is The Electron Shielding Effect? Best 7 Answer What Is An Electron Shielding Effect The orbital (n) and subshell (ml) define how close an electron can. electrons in an atom can shield each other from the pull of the nucleus. the balance between attractive and repulsive forces results in shielding. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. Electrons in an \. What Is An Electron Shielding Effect.
From www.slideserve.com
PPT Chapter 14 Chemical Periodicity PowerPoint Presentation, free What Is An Electron Shielding Effect electrons in an atom can shield each other from the pull of the nucleus. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because of the spherical shape. What Is An Electron Shielding Effect.
From quizizz.com
Chemistry Periodic Law and Electron Shielding Quiz Quizizz What Is An Electron Shielding Effect Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because of the spherical shape of the \ (s\) orbital. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. This effect, called the shielding effect, describes the decrease in. What Is An Electron Shielding Effect.
From www.youtube.com
Shielding Effect and Effective Nuclear Charge YouTube What Is An Electron Shielding Effect electrons in an atom can shield each other from the pull of the nucleus. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. This effect, called the shielding effect, describes the decrease in attraction. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear. What Is An Electron Shielding Effect.