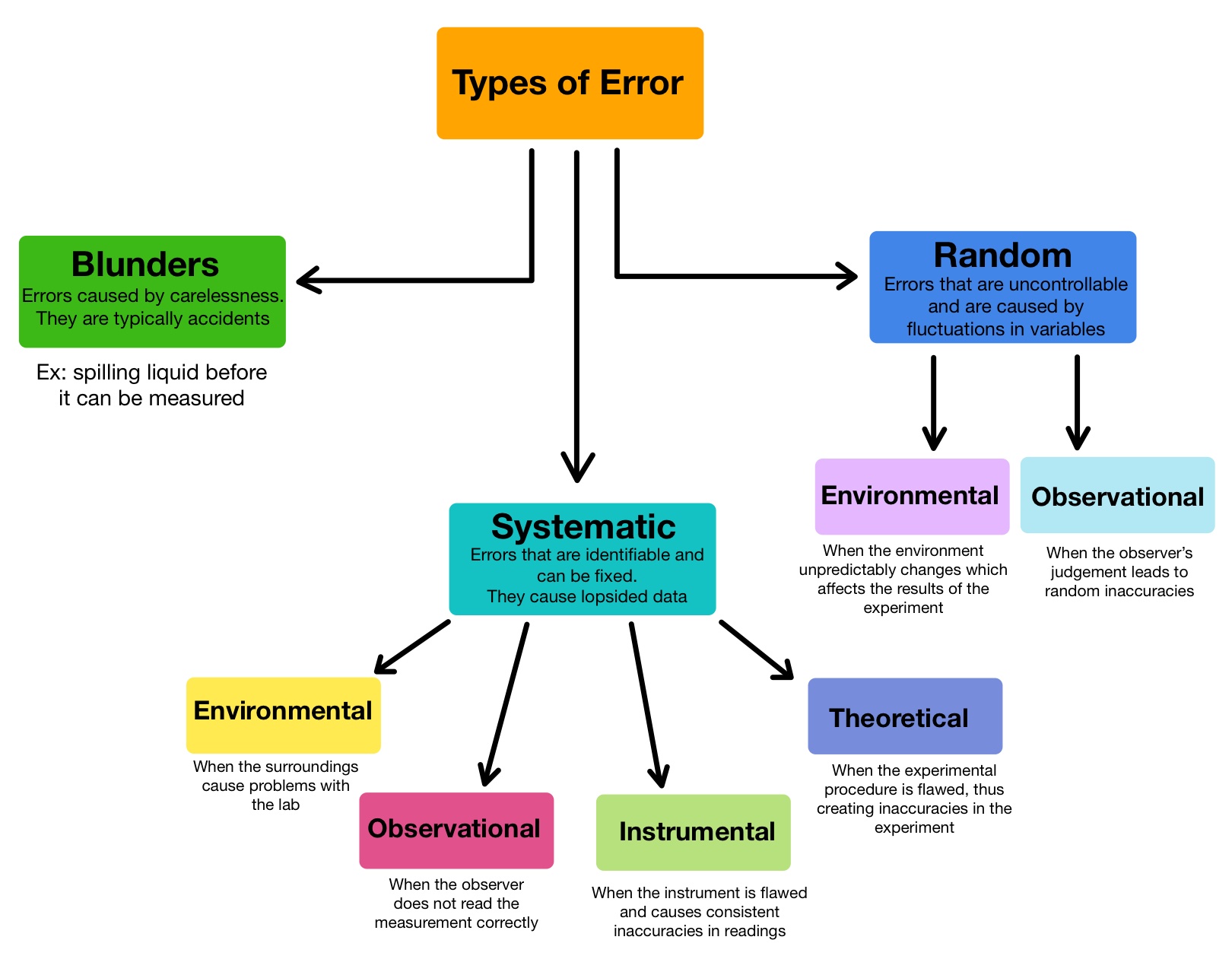
Sources Of Error In Electrochemistry Lab . Learn why all science experiments have error, how to calculate it, and the sources and types of errors you should report. One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads 6.10 when immersed in a ph 6.00 buffer. In electrochemical measurements, errors may arise from a range of sources including the measurement equipment, fluctuations in operating conditions, or. The anode may be less negative, supplying less energy than thermodynamically possible. The cathode is less positive, supplying. Some common sources of error in a flame test lab include contamination of the substance being tested, impurities in the. Check to make sure the electrode frit is not clogged and is immersed in the solution, that no air bubble is close to the frit blocking the solution access to it,. In electrochemical measurements, errors may arise from a range of sources including the measurement equipment, fluctuations in.
from www.expii.com
In electrochemical measurements, errors may arise from a range of sources including the measurement equipment, fluctuations in operating conditions, or. Check to make sure the electrode frit is not clogged and is immersed in the solution, that no air bubble is close to the frit blocking the solution access to it,. In electrochemical measurements, errors may arise from a range of sources including the measurement equipment, fluctuations in. Some common sources of error in a flame test lab include contamination of the substance being tested, impurities in the. Learn why all science experiments have error, how to calculate it, and the sources and types of errors you should report. The cathode is less positive, supplying. One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads 6.10 when immersed in a ph 6.00 buffer. The anode may be less negative, supplying less energy than thermodynamically possible.
Types of Error — Overview & Comparison Expii
Sources Of Error In Electrochemistry Lab The anode may be less negative, supplying less energy than thermodynamically possible. Check to make sure the electrode frit is not clogged and is immersed in the solution, that no air bubble is close to the frit blocking the solution access to it,. One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads 6.10 when immersed in a ph 6.00 buffer. In electrochemical measurements, errors may arise from a range of sources including the measurement equipment, fluctuations in operating conditions, or. Some common sources of error in a flame test lab include contamination of the substance being tested, impurities in the. Learn why all science experiments have error, how to calculate it, and the sources and types of errors you should report. The anode may be less negative, supplying less energy than thermodynamically possible. In electrochemical measurements, errors may arise from a range of sources including the measurement equipment, fluctuations in. The cathode is less positive, supplying.
From cegvzfcp.blob.core.windows.net
Lab Examples Of Error at Edwin Lynn blog Sources Of Error In Electrochemistry Lab Check to make sure the electrode frit is not clogged and is immersed in the solution, that no air bubble is close to the frit blocking the solution access to it,. The anode may be less negative, supplying less energy than thermodynamically possible. One example of a systematic error could be using a ph meter that is incorrectly calibrated, so. Sources Of Error In Electrochemistry Lab.
From felixtrument.ca
Sources of error in lab experiments and laboratory tests Felixtrument Sources Of Error In Electrochemistry Lab Some common sources of error in a flame test lab include contamination of the substance being tested, impurities in the. Learn why all science experiments have error, how to calculate it, and the sources and types of errors you should report. In electrochemical measurements, errors may arise from a range of sources including the measurement equipment, fluctuations in operating conditions,. Sources Of Error In Electrochemistry Lab.
From www.slideserve.com
PPT Experimental Error PowerPoint Presentation, free download ID Sources Of Error In Electrochemistry Lab In electrochemical measurements, errors may arise from a range of sources including the measurement equipment, fluctuations in. Learn why all science experiments have error, how to calculate it, and the sources and types of errors you should report. In electrochemical measurements, errors may arise from a range of sources including the measurement equipment, fluctuations in operating conditions, or. One example. Sources Of Error In Electrochemistry Lab.
From www.scribd.com
Common Sources of Error in Physics Lab Experiments Measuring Sources Of Error In Electrochemistry Lab One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads 6.10 when immersed in a ph 6.00 buffer. Some common sources of error in a flame test lab include contamination of the substance being tested, impurities in the. In electrochemical measurements, errors may arise from a range of sources including. Sources Of Error In Electrochemistry Lab.
From www.semanticscholar.org
Figure 1 from Sources of error in electronic structure calculations on Sources Of Error In Electrochemistry Lab In electrochemical measurements, errors may arise from a range of sources including the measurement equipment, fluctuations in operating conditions, or. One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads 6.10 when immersed in a ph 6.00 buffer. Check to make sure the electrode frit is not clogged and is. Sources Of Error In Electrochemistry Lab.
From www.studypool.com
SOLUTION Sources Of Errors In Moment Lab Studypool Sources Of Error In Electrochemistry Lab Learn why all science experiments have error, how to calculate it, and the sources and types of errors you should report. In electrochemical measurements, errors may arise from a range of sources including the measurement equipment, fluctuations in. Some common sources of error in a flame test lab include contamination of the substance being tested, impurities in the. In electrochemical. Sources Of Error In Electrochemistry Lab.
From itnewstoday.net
The Best Way To Fix Error Sources For A Reaction Rate Lab IT News Today Sources Of Error In Electrochemistry Lab In electrochemical measurements, errors may arise from a range of sources including the measurement equipment, fluctuations in operating conditions, or. Learn why all science experiments have error, how to calculate it, and the sources and types of errors you should report. Some common sources of error in a flame test lab include contamination of the substance being tested, impurities in. Sources Of Error In Electrochemistry Lab.
From www.slideserve.com
PPT QC In Biochemistry PowerPoint Presentation, free download ID Sources Of Error In Electrochemistry Lab The cathode is less positive, supplying. Learn why all science experiments have error, how to calculate it, and the sources and types of errors you should report. In electrochemical measurements, errors may arise from a range of sources including the measurement equipment, fluctuations in operating conditions, or. One example of a systematic error could be using a ph meter that. Sources Of Error In Electrochemistry Lab.
From www.researchgate.net
(PDF) Management of errors in a clinical laboratory Sources Of Error In Electrochemistry Lab The anode may be less negative, supplying less energy than thermodynamically possible. Check to make sure the electrode frit is not clogged and is immersed in the solution, that no air bubble is close to the frit blocking the solution access to it,. Some common sources of error in a flame test lab include contamination of the substance being tested,. Sources Of Error In Electrochemistry Lab.
From www.reddit.com
Technical error in Bard/Faulkner? He reverts the electrochemical Sources Of Error In Electrochemistry Lab Learn why all science experiments have error, how to calculate it, and the sources and types of errors you should report. The cathode is less positive, supplying. Check to make sure the electrode frit is not clogged and is immersed in the solution, that no air bubble is close to the frit blocking the solution access to it,. In electrochemical. Sources Of Error In Electrochemistry Lab.
From www.techyv.com
Learn About Common Sources Of Errors In Physics Labs And Details Sources Of Error In Electrochemistry Lab The anode may be less negative, supplying less energy than thermodynamically possible. The cathode is less positive, supplying. One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads 6.10 when immersed in a ph 6.00 buffer. Check to make sure the electrode frit is not clogged and is immersed in. Sources Of Error In Electrochemistry Lab.
From www.matrix.edu.au
Systematic vs Random Errors in Physics Part 3 of Physics Skills Guide Sources Of Error In Electrochemistry Lab Some common sources of error in a flame test lab include contamination of the substance being tested, impurities in the. One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads 6.10 when immersed in a ph 6.00 buffer. The cathode is less positive, supplying. Learn why all science experiments have. Sources Of Error In Electrochemistry Lab.
From studylib.net
STUDY GUIDE Osmosis/Diffusion, Measurement, Source of Error Sources Of Error In Electrochemistry Lab The anode may be less negative, supplying less energy than thermodynamically possible. The cathode is less positive, supplying. In electrochemical measurements, errors may arise from a range of sources including the measurement equipment, fluctuations in. In electrochemical measurements, errors may arise from a range of sources including the measurement equipment, fluctuations in operating conditions, or. Learn why all science experiments. Sources Of Error In Electrochemistry Lab.
From loerwyfsq.blob.core.windows.net
Sources Of Error In Experiments Examples at Julie Harris blog Sources Of Error In Electrochemistry Lab In electrochemical measurements, errors may arise from a range of sources including the measurement equipment, fluctuations in. The anode may be less negative, supplying less energy than thermodynamically possible. Learn why all science experiments have error, how to calculate it, and the sources and types of errors you should report. Check to make sure the electrode frit is not clogged. Sources Of Error In Electrochemistry Lab.
From cethmxma.blob.core.windows.net
Sources Of Error In Using Spectrophotometer at Mae Eddy blog Sources Of Error In Electrochemistry Lab In electrochemical measurements, errors may arise from a range of sources including the measurement equipment, fluctuations in. Check to make sure the electrode frit is not clogged and is immersed in the solution, that no air bubble is close to the frit blocking the solution access to it,. One example of a systematic error could be using a ph meter. Sources Of Error In Electrochemistry Lab.
From www.expii.com
Types of Error — Overview & Comparison Expii Sources Of Error In Electrochemistry Lab Some common sources of error in a flame test lab include contamination of the substance being tested, impurities in the. Learn why all science experiments have error, how to calculate it, and the sources and types of errors you should report. The cathode is less positive, supplying. In electrochemical measurements, errors may arise from a range of sources including the. Sources Of Error In Electrochemistry Lab.
From www.researchgate.net
The classification of error sources. Download Scientific Diagram Sources Of Error In Electrochemistry Lab In electrochemical measurements, errors may arise from a range of sources including the measurement equipment, fluctuations in. The anode may be less negative, supplying less energy than thermodynamically possible. In electrochemical measurements, errors may arise from a range of sources including the measurement equipment, fluctuations in operating conditions, or. One example of a systematic error could be using a ph. Sources Of Error In Electrochemistry Lab.
From www.slideserve.com
PPT Laboratory Quality Control PowerPoint Presentation ID2424325 Sources Of Error In Electrochemistry Lab The anode may be less negative, supplying less energy than thermodynamically possible. The cathode is less positive, supplying. Some common sources of error in a flame test lab include contamination of the substance being tested, impurities in the. In electrochemical measurements, errors may arise from a range of sources including the measurement equipment, fluctuations in operating conditions, or. Check to. Sources Of Error In Electrochemistry Lab.
From www.researchgate.net
(PDF) Sources of Error in Laboratory Medicine Sources Of Error In Electrochemistry Lab The cathode is less positive, supplying. One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads 6.10 when immersed in a ph 6.00 buffer. Check to make sure the electrode frit is not clogged and is immersed in the solution, that no air bubble is close to the frit blocking. Sources Of Error In Electrochemistry Lab.
From loerwyfsq.blob.core.windows.net
Sources Of Error In Experiments Examples at Julie Harris blog Sources Of Error In Electrochemistry Lab Check to make sure the electrode frit is not clogged and is immersed in the solution, that no air bubble is close to the frit blocking the solution access to it,. In electrochemical measurements, errors may arise from a range of sources including the measurement equipment, fluctuations in operating conditions, or. Some common sources of error in a flame test. Sources Of Error In Electrochemistry Lab.
From www.semanticscholar.org
[PDF] Notizen Potential Error Sources in Combined Electrochemistry Sources Of Error In Electrochemistry Lab The cathode is less positive, supplying. In electrochemical measurements, errors may arise from a range of sources including the measurement equipment, fluctuations in. The anode may be less negative, supplying less energy than thermodynamically possible. One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads 6.10 when immersed in a. Sources Of Error In Electrochemistry Lab.
From erwinnuza.blogspot.com
Types Of Experimental Errors Reasons for Error in a Chemistry Sources Of Error In Electrochemistry Lab The cathode is less positive, supplying. Learn why all science experiments have error, how to calculate it, and the sources and types of errors you should report. One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads 6.10 when immersed in a ph 6.00 buffer. Some common sources of error. Sources Of Error In Electrochemistry Lab.
From www.cs.princeton.edu
Sources of Error Sources Of Error In Electrochemistry Lab Learn why all science experiments have error, how to calculate it, and the sources and types of errors you should report. In electrochemical measurements, errors may arise from a range of sources including the measurement equipment, fluctuations in. The cathode is less positive, supplying. One example of a systematic error could be using a ph meter that is incorrectly calibrated,. Sources Of Error In Electrochemistry Lab.
From writeonline.ca
Write Online Lab Report Writing Guide Resources Sources Of Error In Electrochemistry Lab Learn why all science experiments have error, how to calculate it, and the sources and types of errors you should report. One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads 6.10 when immersed in a ph 6.00 buffer. Some common sources of error in a flame test lab include. Sources Of Error In Electrochemistry Lab.
From www.slideshare.net
Errors in chemical analysis Sources Of Error In Electrochemistry Lab Check to make sure the electrode frit is not clogged and is immersed in the solution, that no air bubble is close to the frit blocking the solution access to it,. The cathode is less positive, supplying. In electrochemical measurements, errors may arise from a range of sources including the measurement equipment, fluctuations in. Learn why all science experiments have. Sources Of Error In Electrochemistry Lab.
From www.youtube.com
Introduction to Error Analysis for Chemistry Lab YouTube Sources Of Error In Electrochemistry Lab In electrochemical measurements, errors may arise from a range of sources including the measurement equipment, fluctuations in. In electrochemical measurements, errors may arise from a range of sources including the measurement equipment, fluctuations in operating conditions, or. One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads 6.10 when immersed. Sources Of Error In Electrochemistry Lab.
From itnewstoday.net
The Best Way To Fix Error Sources For A Reaction Rate Lab IT News Today Sources Of Error In Electrochemistry Lab The cathode is less positive, supplying. One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads 6.10 when immersed in a ph 6.00 buffer. Check to make sure the electrode frit is not clogged and is immersed in the solution, that no air bubble is close to the frit blocking. Sources Of Error In Electrochemistry Lab.
From loerwyfsq.blob.core.windows.net
Sources Of Error In Experiments Examples at Julie Harris blog Sources Of Error In Electrochemistry Lab The anode may be less negative, supplying less energy than thermodynamically possible. In electrochemical measurements, errors may arise from a range of sources including the measurement equipment, fluctuations in. Some common sources of error in a flame test lab include contamination of the substance being tested, impurities in the. Check to make sure the electrode frit is not clogged and. Sources Of Error In Electrochemistry Lab.
From chemistnotes.com
Errors in Chemical Analysis Determinate and Indeterminate Errors Sources Of Error In Electrochemistry Lab The anode may be less negative, supplying less energy than thermodynamically possible. In electrochemical measurements, errors may arise from a range of sources including the measurement equipment, fluctuations in operating conditions, or. Check to make sure the electrode frit is not clogged and is immersed in the solution, that no air bubble is close to the frit blocking the solution. Sources Of Error In Electrochemistry Lab.
From www.youtube.com
Galvanic Cell Sources of Error YouTube Sources Of Error In Electrochemistry Lab In electrochemical measurements, errors may arise from a range of sources including the measurement equipment, fluctuations in operating conditions, or. In electrochemical measurements, errors may arise from a range of sources including the measurement equipment, fluctuations in. One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads 6.10 when immersed. Sources Of Error In Electrochemistry Lab.
From www.slideserve.com
PPT Measurements and Sources of Errors PowerPoint Presentation, free Sources Of Error In Electrochemistry Lab Learn why all science experiments have error, how to calculate it, and the sources and types of errors you should report. Check to make sure the electrode frit is not clogged and is immersed in the solution, that no air bubble is close to the frit blocking the solution access to it,. The anode may be less negative, supplying less. Sources Of Error In Electrochemistry Lab.
From ohmlaw.com
5 Error Sources in Ohm's Law Experiment [How to avoid them] • Ohm Law Sources Of Error In Electrochemistry Lab In electrochemical measurements, errors may arise from a range of sources including the measurement equipment, fluctuations in. The anode may be less negative, supplying less energy than thermodynamically possible. One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads 6.10 when immersed in a ph 6.00 buffer. Some common sources. Sources Of Error In Electrochemistry Lab.
From www.slideserve.com
PPT Errors and Uncertainties PowerPoint Presentation, free download Sources Of Error In Electrochemistry Lab Check to make sure the electrode frit is not clogged and is immersed in the solution, that no air bubble is close to the frit blocking the solution access to it,. In electrochemical measurements, errors may arise from a range of sources including the measurement equipment, fluctuations in operating conditions, or. In electrochemical measurements, errors may arise from a range. Sources Of Error In Electrochemistry Lab.
From www.expii.com
Types of Error — Overview & Comparison Expii Sources Of Error In Electrochemistry Lab Check to make sure the electrode frit is not clogged and is immersed in the solution, that no air bubble is close to the frit blocking the solution access to it,. In electrochemical measurements, errors may arise from a range of sources including the measurement equipment, fluctuations in operating conditions, or. Some common sources of error in a flame test. Sources Of Error In Electrochemistry Lab.
From www.youtube.com
Common Errors in Laboratory Reports YouTube Sources Of Error In Electrochemistry Lab Check to make sure the electrode frit is not clogged and is immersed in the solution, that no air bubble is close to the frit blocking the solution access to it,. One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads 6.10 when immersed in a ph 6.00 buffer. In. Sources Of Error In Electrochemistry Lab.