What Is Energy Equilibrium . Learn how to use energy diagrams to describe the motion of an object with constant total mechanical energy and a potential energy function. “the condition under which two objects in physical contact with each other exchanges no heat energy is termed as thermal. To know the relationship between free energy and the equilibrium constant. Find out how to identify stable and unstable equilibrium points and how to visualize the motion as a marble rolling down the potential energy function. Equilibrium thermodynamics is the systematic study of transformations of matter and energy in systems in terms of a concept called. The sign of the standard free energy change δ g ° of a chemical reaction determines whether the reaction will tend to proceed in the forward or reverse direction. Learn how to relate the standard free energy of reaction (\\delta g^o) and the equilibrium constant (k) for gases and liquids. Learn how to calculate and interpret δg° and k for chemical reactions, and how they depend on temperature and entropy. By definition, this equilibrium energy is the one that gives temperature equality between the subsystem and the reservoir, t (e ¯, n, v) = t 1.
from www.sciencefacts.net
Find out how to identify stable and unstable equilibrium points and how to visualize the motion as a marble rolling down the potential energy function. Learn how to calculate and interpret δg° and k for chemical reactions, and how they depend on temperature and entropy. Learn how to relate the standard free energy of reaction (\\delta g^o) and the equilibrium constant (k) for gases and liquids. “the condition under which two objects in physical contact with each other exchanges no heat energy is termed as thermal. Learn how to use energy diagrams to describe the motion of an object with constant total mechanical energy and a potential energy function. The sign of the standard free energy change δ g ° of a chemical reaction determines whether the reaction will tend to proceed in the forward or reverse direction. Equilibrium thermodynamics is the systematic study of transformations of matter and energy in systems in terms of a concept called. To know the relationship between free energy and the equilibrium constant. By definition, this equilibrium energy is the one that gives temperature equality between the subsystem and the reservoir, t (e ¯, n, v) = t 1.
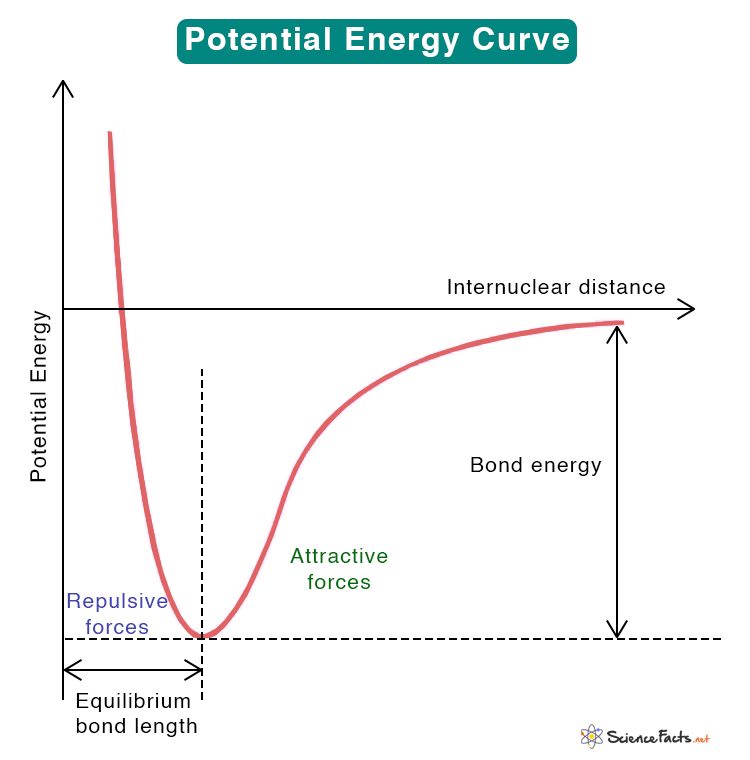
Potential Energy Curve
What Is Energy Equilibrium The sign of the standard free energy change δ g ° of a chemical reaction determines whether the reaction will tend to proceed in the forward or reverse direction. Equilibrium thermodynamics is the systematic study of transformations of matter and energy in systems in terms of a concept called. The sign of the standard free energy change δ g ° of a chemical reaction determines whether the reaction will tend to proceed in the forward or reverse direction. Learn how to relate the standard free energy of reaction (\\delta g^o) and the equilibrium constant (k) for gases and liquids. By definition, this equilibrium energy is the one that gives temperature equality between the subsystem and the reservoir, t (e ¯, n, v) = t 1. “the condition under which two objects in physical contact with each other exchanges no heat energy is termed as thermal. Find out how to identify stable and unstable equilibrium points and how to visualize the motion as a marble rolling down the potential energy function. Learn how to calculate and interpret δg° and k for chemical reactions, and how they depend on temperature and entropy. To know the relationship between free energy and the equilibrium constant. Learn how to use energy diagrams to describe the motion of an object with constant total mechanical energy and a potential energy function.
From www.researchgate.net
Equilibrium coexistence of ice and water. Illustration of potential What Is Energy Equilibrium Find out how to identify stable and unstable equilibrium points and how to visualize the motion as a marble rolling down the potential energy function. Learn how to use energy diagrams to describe the motion of an object with constant total mechanical energy and a potential energy function. “the condition under which two objects in physical contact with each other. What Is Energy Equilibrium.
From www.ck12.org
Calculations of Free Energy and Keq CK12 Foundation What Is Energy Equilibrium To know the relationship between free energy and the equilibrium constant. Learn how to calculate and interpret δg° and k for chemical reactions, and how they depend on temperature and entropy. Learn how to relate the standard free energy of reaction (\\delta g^o) and the equilibrium constant (k) for gases and liquids. The sign of the standard free energy change. What Is Energy Equilibrium.
From www.online-sciences.com
Chemical Equilibrium, Chemical reactions types, complete reactions and What Is Energy Equilibrium Equilibrium thermodynamics is the systematic study of transformations of matter and energy in systems in terms of a concept called. Learn how to use energy diagrams to describe the motion of an object with constant total mechanical energy and a potential energy function. By definition, this equilibrium energy is the one that gives temperature equality between the subsystem and the. What Is Energy Equilibrium.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID What Is Energy Equilibrium To know the relationship between free energy and the equilibrium constant. The sign of the standard free energy change δ g ° of a chemical reaction determines whether the reaction will tend to proceed in the forward or reverse direction. By definition, this equilibrium energy is the one that gives temperature equality between the subsystem and the reservoir, t (e. What Is Energy Equilibrium.
From www.slideserve.com
PPT Free Energy and Equilibrium PowerPoint Presentation, free What Is Energy Equilibrium Equilibrium thermodynamics is the systematic study of transformations of matter and energy in systems in terms of a concept called. “the condition under which two objects in physical contact with each other exchanges no heat energy is termed as thermal. Learn how to use energy diagrams to describe the motion of an object with constant total mechanical energy and a. What Is Energy Equilibrium.
From www.youtube.com
Statement I For the stable equilibrium force has to be zero and What Is Energy Equilibrium Learn how to relate the standard free energy of reaction (\\delta g^o) and the equilibrium constant (k) for gases and liquids. By definition, this equilibrium energy is the one that gives temperature equality between the subsystem and the reservoir, t (e ¯, n, v) = t 1. Learn how to use energy diagrams to describe the motion of an object. What Is Energy Equilibrium.
From app.jove.com
Free Energy and Equilibrium Concept Cell Biology JoVe What Is Energy Equilibrium By definition, this equilibrium energy is the one that gives temperature equality between the subsystem and the reservoir, t (e ¯, n, v) = t 1. Learn how to use energy diagrams to describe the motion of an object with constant total mechanical energy and a potential energy function. Learn how to relate the standard free energy of reaction (\\delta. What Is Energy Equilibrium.
From www.slideserve.com
PPT Energy Changes in Chemical Reactions Chapter 17 PowerPoint What Is Energy Equilibrium “the condition under which two objects in physical contact with each other exchanges no heat energy is termed as thermal. Find out how to identify stable and unstable equilibrium points and how to visualize the motion as a marble rolling down the potential energy function. Learn how to calculate and interpret δg° and k for chemical reactions, and how they. What Is Energy Equilibrium.
From www.youtube.com
Free Energy, Equilibrium Constant, and Cell Potential Relationships What Is Energy Equilibrium Learn how to calculate and interpret δg° and k for chemical reactions, and how they depend on temperature and entropy. By definition, this equilibrium energy is the one that gives temperature equality between the subsystem and the reservoir, t (e ¯, n, v) = t 1. Learn how to use energy diagrams to describe the motion of an object with. What Is Energy Equilibrium.
From www.slideserve.com
PPT Free Energy and Equilibrium PowerPoint Presentation, free What Is Energy Equilibrium To know the relationship between free energy and the equilibrium constant. Learn how to use energy diagrams to describe the motion of an object with constant total mechanical energy and a potential energy function. Find out how to identify stable and unstable equilibrium points and how to visualize the motion as a marble rolling down the potential energy function. “the. What Is Energy Equilibrium.
From study.com
Finding Equilibrium Bond Length from a Graph Chemistry What Is Energy Equilibrium The sign of the standard free energy change δ g ° of a chemical reaction determines whether the reaction will tend to proceed in the forward or reverse direction. Learn how to relate the standard free energy of reaction (\\delta g^o) and the equilibrium constant (k) for gases and liquids. Learn how to calculate and interpret δg° and k for. What Is Energy Equilibrium.
From www.sciencefacts.net
Potential Energy Curve What Is Energy Equilibrium Learn how to relate the standard free energy of reaction (\\delta g^o) and the equilibrium constant (k) for gases and liquids. Equilibrium thermodynamics is the systematic study of transformations of matter and energy in systems in terms of a concept called. Find out how to identify stable and unstable equilibrium points and how to visualize the motion as a marble. What Is Energy Equilibrium.
From www.slideserve.com
PPT Gibbs Free Energy PowerPoint Presentation, free download ID6125811 What Is Energy Equilibrium Learn how to use energy diagrams to describe the motion of an object with constant total mechanical energy and a potential energy function. The sign of the standard free energy change δ g ° of a chemical reaction determines whether the reaction will tend to proceed in the forward or reverse direction. By definition, this equilibrium energy is the one. What Is Energy Equilibrium.
From study.com
Delta G & Equilibrium Constant Formula & Examples Lesson What Is Energy Equilibrium By definition, this equilibrium energy is the one that gives temperature equality between the subsystem and the reservoir, t (e ¯, n, v) = t 1. To know the relationship between free energy and the equilibrium constant. Equilibrium thermodynamics is the systematic study of transformations of matter and energy in systems in terms of a concept called. Learn how to. What Is Energy Equilibrium.
From general.chemistrysteps.com
Cell Potential, Free Energy, and Equilibrium Constant Chemistry Steps What Is Energy Equilibrium To know the relationship between free energy and the equilibrium constant. By definition, this equilibrium energy is the one that gives temperature equality between the subsystem and the reservoir, t (e ¯, n, v) = t 1. Learn how to calculate and interpret δg° and k for chemical reactions, and how they depend on temperature and entropy. Learn how to. What Is Energy Equilibrium.
From www.slideserve.com
PPT Chapter 18 Entropy, Free Energy, and Equilibrium PowerPoint What Is Energy Equilibrium To know the relationship between free energy and the equilibrium constant. Find out how to identify stable and unstable equilibrium points and how to visualize the motion as a marble rolling down the potential energy function. Learn how to calculate and interpret δg° and k for chemical reactions, and how they depend on temperature and entropy. Learn how to use. What Is Energy Equilibrium.
From www.youtube.com
Thermodynamics Free Energy, Pressure & Equilibrium. YouTube What Is Energy Equilibrium Learn how to relate the standard free energy of reaction (\\delta g^o) and the equilibrium constant (k) for gases and liquids. Equilibrium thermodynamics is the systematic study of transformations of matter and energy in systems in terms of a concept called. To know the relationship between free energy and the equilibrium constant. By definition, this equilibrium energy is the one. What Is Energy Equilibrium.
From www.youtube.com
Gibbs free energy and equilibrium YouTube What Is Energy Equilibrium To know the relationship between free energy and the equilibrium constant. Learn how to relate the standard free energy of reaction (\\delta g^o) and the equilibrium constant (k) for gases and liquids. Equilibrium thermodynamics is the systematic study of transformations of matter and energy in systems in terms of a concept called. “the condition under which two objects in physical. What Is Energy Equilibrium.
From mavink.com
What Is K In Gibbs Free Energy What Is Energy Equilibrium Learn how to calculate and interpret δg° and k for chemical reactions, and how they depend on temperature and entropy. Learn how to relate the standard free energy of reaction (\\delta g^o) and the equilibrium constant (k) for gases and liquids. To know the relationship between free energy and the equilibrium constant. By definition, this equilibrium energy is the one. What Is Energy Equilibrium.
From scienceinfo.com
Free Energy and Equilibrium Constant What Is Energy Equilibrium Learn how to use energy diagrams to describe the motion of an object with constant total mechanical energy and a potential energy function. To know the relationship between free energy and the equilibrium constant. Learn how to calculate and interpret δg° and k for chemical reactions, and how they depend on temperature and entropy. By definition, this equilibrium energy is. What Is Energy Equilibrium.
From www.slideserve.com
PPT Gibbs Free Energy PowerPoint Presentation, free download ID6125811 What Is Energy Equilibrium Learn how to use energy diagrams to describe the motion of an object with constant total mechanical energy and a potential energy function. By definition, this equilibrium energy is the one that gives temperature equality between the subsystem and the reservoir, t (e ¯, n, v) = t 1. Learn how to relate the standard free energy of reaction (\\delta. What Is Energy Equilibrium.
From www.chemicals.co.uk
Chemistry A Level Revision Equilibrium What Is Energy Equilibrium Learn how to relate the standard free energy of reaction (\\delta g^o) and the equilibrium constant (k) for gases and liquids. “the condition under which two objects in physical contact with each other exchanges no heat energy is termed as thermal. To know the relationship between free energy and the equilibrium constant. Learn how to use energy diagrams to describe. What Is Energy Equilibrium.
From www.youtube.com
W02M04 Dynamic equilibrium equation by energy method YouTube What Is Energy Equilibrium To know the relationship between free energy and the equilibrium constant. “the condition under which two objects in physical contact with each other exchanges no heat energy is termed as thermal. Learn how to calculate and interpret δg° and k for chemical reactions, and how they depend on temperature and entropy. Learn how to relate the standard free energy of. What Is Energy Equilibrium.
From www.investopedia.com
Equilibrium Quantity Definition What Is Energy Equilibrium “the condition under which two objects in physical contact with each other exchanges no heat energy is termed as thermal. The sign of the standard free energy change δ g ° of a chemical reaction determines whether the reaction will tend to proceed in the forward or reverse direction. Equilibrium thermodynamics is the systematic study of transformations of matter and. What Is Energy Equilibrium.
From www.researchgate.net
1 Schematic plot of Gibbs free energy versus progress of reaction. The What Is Energy Equilibrium By definition, this equilibrium energy is the one that gives temperature equality between the subsystem and the reservoir, t (e ¯, n, v) = t 1. Learn how to calculate and interpret δg° and k for chemical reactions, and how they depend on temperature and entropy. Equilibrium thermodynamics is the systematic study of transformations of matter and energy in systems. What Is Energy Equilibrium.
From www.youtube.com
17.1 Equilibrium and Gibbs free energy (HL) YouTube What Is Energy Equilibrium To know the relationship between free energy and the equilibrium constant. The sign of the standard free energy change δ g ° of a chemical reaction determines whether the reaction will tend to proceed in the forward or reverse direction. “the condition under which two objects in physical contact with each other exchanges no heat energy is termed as thermal.. What Is Energy Equilibrium.
From www.bartleby.com
Answered Based on the Potential Energy vs… bartleby What Is Energy Equilibrium Learn how to relate the standard free energy of reaction (\\delta g^o) and the equilibrium constant (k) for gases and liquids. The sign of the standard free energy change δ g ° of a chemical reaction determines whether the reaction will tend to proceed in the forward or reverse direction. Learn how to use energy diagrams to describe the motion. What Is Energy Equilibrium.
From www.chegg.com
Solved For the potential energy graph below, determine the What Is Energy Equilibrium Learn how to use energy diagrams to describe the motion of an object with constant total mechanical energy and a potential energy function. The sign of the standard free energy change δ g ° of a chemical reaction determines whether the reaction will tend to proceed in the forward or reverse direction. Equilibrium thermodynamics is the systematic study of transformations. What Is Energy Equilibrium.
From www.slideserve.com
PPT Packet 17 Free Energy and Thermodynamics PowerPoint Presentation What Is Energy Equilibrium The sign of the standard free energy change δ g ° of a chemical reaction determines whether the reaction will tend to proceed in the forward or reverse direction. Find out how to identify stable and unstable equilibrium points and how to visualize the motion as a marble rolling down the potential energy function. Learn how to relate the standard. What Is Energy Equilibrium.
From www.youtube.com
Gibbs Free Energy Entropy, Enthalpy & Equilibrium Constant K YouTube What Is Energy Equilibrium Find out how to identify stable and unstable equilibrium points and how to visualize the motion as a marble rolling down the potential energy function. “the condition under which two objects in physical contact with each other exchanges no heat energy is termed as thermal. By definition, this equilibrium energy is the one that gives temperature equality between the subsystem. What Is Energy Equilibrium.
From www.slideserve.com
PPT First Law of Thermodynamics PowerPoint Presentation, free What Is Energy Equilibrium Equilibrium thermodynamics is the systematic study of transformations of matter and energy in systems in terms of a concept called. Learn how to use energy diagrams to describe the motion of an object with constant total mechanical energy and a potential energy function. Find out how to identify stable and unstable equilibrium points and how to visualize the motion as. What Is Energy Equilibrium.
From facts.net
18 Unbelievable Facts About Dynamic Equilibrium What Is Energy Equilibrium By definition, this equilibrium energy is the one that gives temperature equality between the subsystem and the reservoir, t (e ¯, n, v) = t 1. Find out how to identify stable and unstable equilibrium points and how to visualize the motion as a marble rolling down the potential energy function. Equilibrium thermodynamics is the systematic study of transformations of. What Is Energy Equilibrium.
From www.ck12.org
Relationship between Free Energy and Equilibrium Constant Overview What Is Energy Equilibrium Equilibrium thermodynamics is the systematic study of transformations of matter and energy in systems in terms of a concept called. By definition, this equilibrium energy is the one that gives temperature equality between the subsystem and the reservoir, t (e ¯, n, v) = t 1. “the condition under which two objects in physical contact with each other exchanges no. What Is Energy Equilibrium.
From www.slideshare.net
IB Chemistry on Gibbs Free Energy, Equilibrium constant and Cell Pote… What Is Energy Equilibrium “the condition under which two objects in physical contact with each other exchanges no heat energy is termed as thermal. Equilibrium thermodynamics is the systematic study of transformations of matter and energy in systems in terms of a concept called. The sign of the standard free energy change δ g ° of a chemical reaction determines whether the reaction will. What Is Energy Equilibrium.
From www.slideserve.com
PPT Chapters 6, 7 Energy PowerPoint Presentation, free download ID What Is Energy Equilibrium The sign of the standard free energy change δ g ° of a chemical reaction determines whether the reaction will tend to proceed in the forward or reverse direction. Learn how to calculate and interpret δg° and k for chemical reactions, and how they depend on temperature and entropy. To know the relationship between free energy and the equilibrium constant.. What Is Energy Equilibrium.