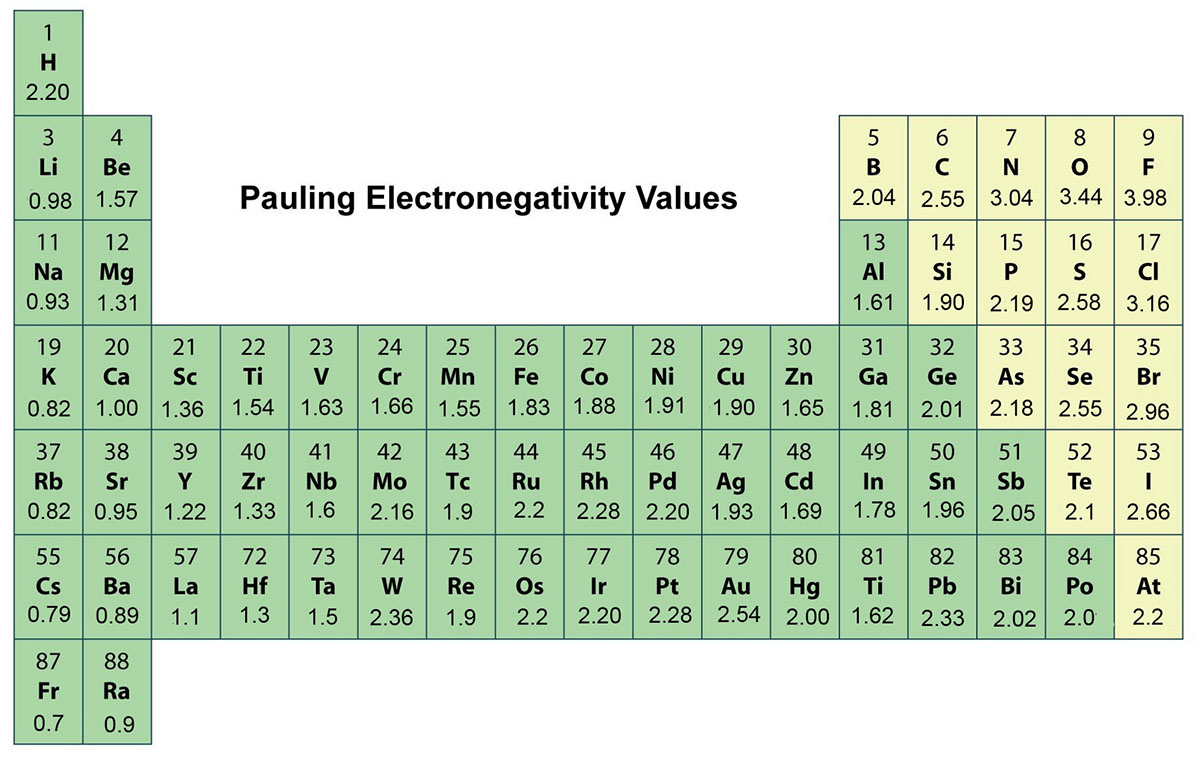
Electronegativity Chlorine Fluorine . Knowing that fluorine is the most electronegative element and arbitrarily setting its electronegativity to 4, pauling obtained. As you go down a group, electronegativity decreases because the bonding pair of electrons is increasingly distant from the attraction of the nucleus. Fluorine is the most electronegative element on the periodic table. It can also be used to predict if the resulting molecule will be polar. It is found in the 7th group and 2nd period of the periodic table and. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. Fluorine (the most electronegative element) is assigned a value of 4.0, and values range down to caesium and francium which are the least. Both of these trends show that fluorine is highly electronegative (it pulls a shared pair of bonding electrons towards itself). Its electronegativity value is 3.98. The pauling scale is the most.
from www.nanowerk.com
As you go down a group, electronegativity decreases because the bonding pair of electrons is increasingly distant from the attraction of the nucleus. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. Fluorine is the most electronegative element on the periodic table. Both of these trends show that fluorine is highly electronegative (it pulls a shared pair of bonding electrons towards itself). It is found in the 7th group and 2nd period of the periodic table and. Fluorine (the most electronegative element) is assigned a value of 4.0, and values range down to caesium and francium which are the least. Knowing that fluorine is the most electronegative element and arbitrarily setting its electronegativity to 4, pauling obtained. It can also be used to predict if the resulting molecule will be polar. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. Its electronegativity value is 3.98.
Electronegativity explained
Electronegativity Chlorine Fluorine As you go down a group, electronegativity decreases because the bonding pair of electrons is increasingly distant from the attraction of the nucleus. It is found in the 7th group and 2nd period of the periodic table and. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. It can also be used to predict if the resulting molecule will be polar. Both of these trends show that fluorine is highly electronegative (it pulls a shared pair of bonding electrons towards itself). Fluorine is the most electronegative element on the periodic table. The pauling scale is the most. Knowing that fluorine is the most electronegative element and arbitrarily setting its electronegativity to 4, pauling obtained. Its electronegativity value is 3.98. As you go down a group, electronegativity decreases because the bonding pair of electrons is increasingly distant from the attraction of the nucleus. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. Fluorine (the most electronegative element) is assigned a value of 4.0, and values range down to caesium and francium which are the least.
From www.thoughtco.com
Printable Periodic Table of the Elements Electronegativity Electronegativity Chlorine Fluorine Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. The pauling scale is the most. Its electronegativity value is 3.98. As you go down a group, electronegativity decreases because the bonding pair of electrons is increasingly distant from the attraction of the nucleus. Fluorine (the most electronegative element) is assigned a value. Electronegativity Chlorine Fluorine.
From www.numerade.com
SOLVED Sodium (Na) and hydrogen (H) have the same number of electrons Electronegativity Chlorine Fluorine Both of these trends show that fluorine is highly electronegative (it pulls a shared pair of bonding electrons towards itself). It can also be used to predict if the resulting molecule will be polar. Fluorine (the most electronegative element) is assigned a value of 4.0, and values range down to caesium and francium which are the least. Its electronegativity value. Electronegativity Chlorine Fluorine.
From www.nanowerk.com
Electronegativity explained Electronegativity Chlorine Fluorine The pauling scale is the most. Fluorine (the most electronegative element) is assigned a value of 4.0, and values range down to caesium and francium which are the least. Knowing that fluorine is the most electronegative element and arbitrarily setting its electronegativity to 4, pauling obtained. Electronegativity is a measure of the tendency of an atom to attract a bonding. Electronegativity Chlorine Fluorine.
From valenceelectrons.com
Fluorine Electron Configuration With Full Orbital Diagram Electronegativity Chlorine Fluorine The pauling scale is the most. It can also be used to predict if the resulting molecule will be polar. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. Fluorine is the most electronegative element on the periodic table. Its electronegativity value is 3.98. It is found in the 7th group and. Electronegativity Chlorine Fluorine.
From www.youtube.com
Chlorine has ___________ electron affinity than fluorine. YouTube Electronegativity Chlorine Fluorine Fluorine (the most electronegative element) is assigned a value of 4.0, and values range down to caesium and francium which are the least. It is found in the 7th group and 2nd period of the periodic table and. Its electronegativity value is 3.98. The pauling scale is the most. Both of these trends show that fluorine is highly electronegative (it. Electronegativity Chlorine Fluorine.
From mungfali.com
Atom Electronegativity Chart Electronegativity Chlorine Fluorine Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. It is found in the 7th group and 2nd period of the periodic table and. It can also be used to predict if the resulting molecule will be polar. Knowing that fluorine is the most electronegative element and arbitrarily setting its electronegativity to. Electronegativity Chlorine Fluorine.
From material-properties.org
Sulfur and Chlorine Comparison Properties Material Properties Electronegativity Chlorine Fluorine Fluorine (the most electronegative element) is assigned a value of 4.0, and values range down to caesium and francium which are the least. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. As you go down a group, electronegativity decreases because the bonding pair of electrons is increasingly distant from the attraction. Electronegativity Chlorine Fluorine.
From byjus.com
why fluorine is more electronegative than oxygen. Electronegativity Chlorine Fluorine Knowing that fluorine is the most electronegative element and arbitrarily setting its electronegativity to 4, pauling obtained. Its electronegativity value is 3.98. It is found in the 7th group and 2nd period of the periodic table and. It can also be used to predict if the resulting molecule will be polar. Fluorine (the most electronegative element) is assigned a value. Electronegativity Chlorine Fluorine.
From slideplayer.com
Ionization Energy and Electron Affinity ppt download Electronegativity Chlorine Fluorine 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. The pauling scale is the most. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. Fluorine (the most electronegative element) is assigned a value of 4.0, and values range down to caesium and francium which. Electronegativity Chlorine Fluorine.
From iperiodictable.com
What is Electronegativity Chart List of Electronegativity [PDF Electronegativity Chlorine Fluorine Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. As you go down a group, electronegativity decreases because the bonding pair of electrons is increasingly distant from the attraction of the nucleus. Fluorine is the most electronegative element on the periodic table. Fluorine (the most electronegative element) is assigned a value of. Electronegativity Chlorine Fluorine.
From www.bigstockphoto.com
Electronegativity Image & Photo (Free Trial) Bigstock Electronegativity Chlorine Fluorine Knowing that fluorine is the most electronegative element and arbitrarily setting its electronegativity to 4, pauling obtained. Both of these trends show that fluorine is highly electronegative (it pulls a shared pair of bonding electrons towards itself). It can also be used to predict if the resulting molecule will be polar. It is found in the 7th group and 2nd. Electronegativity Chlorine Fluorine.
From material-properties.org
Hydrogen and Fluorine Comparison Properties Material Properties Electronegativity Chlorine Fluorine The pauling scale is the most. As you go down a group, electronegativity decreases because the bonding pair of electrons is increasingly distant from the attraction of the nucleus. It is found in the 7th group and 2nd period of the periodic table and. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or. Electronegativity Chlorine Fluorine.
From www.chemistrylearner.com
Electronegativity Definition, Value Chart, and Trend in Periodic Table Electronegativity Chlorine Fluorine It is found in the 7th group and 2nd period of the periodic table and. As you go down a group, electronegativity decreases because the bonding pair of electrons is increasingly distant from the attraction of the nucleus. Fluorine (the most electronegative element) is assigned a value of 4.0, and values range down to caesium and francium which are the. Electronegativity Chlorine Fluorine.
From www.slideserve.com
PPT Periodic Table PowerPoint Presentation, free download ID5631786 Electronegativity Chlorine Fluorine Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. The pauling scale is the most. Fluorine is the most electronegative element on the periodic table. It is found in the 7th group and 2nd period of the periodic table and. It can also be used to predict if the resulting molecule will. Electronegativity Chlorine Fluorine.
From www.slideserve.com
PPT AS Chemistry PowerPoint Presentation ID2093824 Electronegativity Chlorine Fluorine It is found in the 7th group and 2nd period of the periodic table and. Fluorine (the most electronegative element) is assigned a value of 4.0, and values range down to caesium and francium which are the least. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. Knowing that fluorine is the. Electronegativity Chlorine Fluorine.
From www.dreamstime.com
Fluorine Chemical Element with 9 Atomic Number, Atomic Mass and Electronegativity Chlorine Fluorine The pauling scale is the most. It can also be used to predict if the resulting molecule will be polar. Fluorine is the most electronegative element on the periodic table. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. It is found in the 7th group and 2nd period of the periodic. Electronegativity Chlorine Fluorine.
From www.numerade.com
SOLVED The following chart shows the electronegativity of four Electronegativity Chlorine Fluorine Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. Its electronegativity value is 3.98. Both of these trends show that fluorine is highly electronegative (it pulls a shared pair of bonding electrons towards itself). Knowing. Electronegativity Chlorine Fluorine.
From www.toppr.com
The electronegativity of fluorine obtained from the following data is x Electronegativity Chlorine Fluorine It can also be used to predict if the resulting molecule will be polar. As you go down a group, electronegativity decreases because the bonding pair of electrons is increasingly distant from the attraction of the nucleus. Knowing that fluorine is the most electronegative element and arbitrarily setting its electronegativity to 4, pauling obtained. Fluorine is the most electronegative element. Electronegativity Chlorine Fluorine.
From www.slideshare.net
Electronegativity part two Electronegativity Chlorine Fluorine As you go down a group, electronegativity decreases because the bonding pair of electrons is increasingly distant from the attraction of the nucleus. It is found in the 7th group and 2nd period of the periodic table and. The pauling scale is the most. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of. Electronegativity Chlorine Fluorine.
From www.nuclear-power.com
Fluorine Electron Affinity Electronegativity Ionization Energy of Electronegativity Chlorine Fluorine Both of these trends show that fluorine is highly electronegative (it pulls a shared pair of bonding electrons towards itself). Its electronegativity value is 3.98. It can also be used to predict if the resulting molecule will be polar. It is found in the 7th group and 2nd period of the periodic table and. Fluorine is the most electronegative element. Electronegativity Chlorine Fluorine.
From fluxdesignhouse.blogspot.com
How To Find Electronegativity Of An Element fluxdesignhouse Electronegativity Chlorine Fluorine Both of these trends show that fluorine is highly electronegative (it pulls a shared pair of bonding electrons towards itself). 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. Knowing that fluorine is the most electronegative element and arbitrarily setting its electronegativity to 4, pauling obtained. As you go down a group,. Electronegativity Chlorine Fluorine.
From www.gauthmath.com
Which element is most likely to react with chlorine Electronegativity Chlorine Fluorine Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. Fluorine is the most electronegative element on the periodic table. As you go down a group, electronegativity decreases because the bonding pair of electrons is increasingly distant from the attraction of the nucleus. It is found in the 7th group and 2nd period. Electronegativity Chlorine Fluorine.
From www.slideserve.com
PPT Why is Fluorine more electronegative than Carbon? PowerPoint Electronegativity Chlorine Fluorine The pauling scale is the most. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. It can also be used to predict if the resulting molecule will be polar. Its electronegativity value is 3.98. As you go down a group, electronegativity decreases because the bonding pair of electrons is increasingly distant from. Electronegativity Chlorine Fluorine.
From www.toppr.com
Electronegativity of chlorine is three. Electron affinity of chlorine Electronegativity Chlorine Fluorine Fluorine (the most electronegative element) is assigned a value of 4.0, and values range down to caesium and francium which are the least. The pauling scale is the most. Both of these trends show that fluorine is highly electronegative (it pulls a shared pair of bonding electrons towards itself). 119 rows electronegativity is used to predict whether a bond between. Electronegativity Chlorine Fluorine.
From www.dreamstime.com
Fluorine Chemical Element with First Ionization Energy, Atomic Mass and Electronegativity Chlorine Fluorine The pauling scale is the most. Both of these trends show that fluorine is highly electronegative (it pulls a shared pair of bonding electrons towards itself). Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. Fluorine (the most electronegative element) is assigned a value of 4.0, and values range down to caesium. Electronegativity Chlorine Fluorine.
From www.animalia-life.club
Electronegativity Periodic Table 3d Electronegativity Chlorine Fluorine Knowing that fluorine is the most electronegative element and arbitrarily setting its electronegativity to 4, pauling obtained. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. Both of these trends show that fluorine is highly electronegative (it pulls a shared pair of bonding electrons towards itself). Fluorine is the most electronegative element. Electronegativity Chlorine Fluorine.
From material-properties.org
Fluorine Periodic Table and Atomic Properties Electronegativity Chlorine Fluorine It is found in the 7th group and 2nd period of the periodic table and. Both of these trends show that fluorine is highly electronegative (it pulls a shared pair of bonding electrons towards itself). Fluorine (the most electronegative element) is assigned a value of 4.0, and values range down to caesium and francium which are the least. Its electronegativity. Electronegativity Chlorine Fluorine.
From www.numerade.com
SOLVED PLEASE HELP 15 POINTS AND MARKED BRAINLIST... Use the chart to Electronegativity Chlorine Fluorine Its electronegativity value is 3.98. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. It is found in the 7th group and 2nd period of the periodic table and. Fluorine is the most electronegative element on the periodic table. Fluorine (the most electronegative element) is assigned a value of 4.0, and values. Electronegativity Chlorine Fluorine.
From nl.dreamstime.com
Electronegativity Periodieke Lijst Stock Illustratie Illustration of Electronegativity Chlorine Fluorine Fluorine (the most electronegative element) is assigned a value of 4.0, and values range down to caesium and francium which are the least. The pauling scale is the most. It can also be used to predict if the resulting molecule will be polar. Both of these trends show that fluorine is highly electronegative (it pulls a shared pair of bonding. Electronegativity Chlorine Fluorine.
From periodictable.me
How To Find Electron Configuration For Fluorine Dynamic Periodic Electronegativity Chlorine Fluorine As you go down a group, electronegativity decreases because the bonding pair of electrons is increasingly distant from the attraction of the nucleus. Its electronegativity value is 3.98. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. It can also be used to predict if the resulting molecule will be polar. Electronegativity. Electronegativity Chlorine Fluorine.
From www.vedantu.com
Going from fluorine, chlorine, bromine to iodine the electronegativity Electronegativity Chlorine Fluorine Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. Its electronegativity value is 3.98. Fluorine (the most electronegative element) is assigned a value of 4.0, and values range down to caesium and francium which are. Electronegativity Chlorine Fluorine.
From slideplayer.com
C and C have the same electronegativity. ppt download Electronegativity Chlorine Fluorine It can also be used to predict if the resulting molecule will be polar. The pauling scale is the most. Its electronegativity value is 3.98. As you go down a group, electronegativity decreases because the bonding pair of electrons is increasingly distant from the attraction of the nucleus. Fluorine is the most electronegative element on the periodic table. It is. Electronegativity Chlorine Fluorine.
From askfilo.com
If the electronegativities of fluorine and chlorine are 4.0 and 3.0 resp.. Electronegativity Chlorine Fluorine It is found in the 7th group and 2nd period of the periodic table and. Both of these trends show that fluorine is highly electronegative (it pulls a shared pair of bonding electrons towards itself). As you go down a group, electronegativity decreases because the bonding pair of electrons is increasingly distant from the attraction of the nucleus. Electronegativity is. Electronegativity Chlorine Fluorine.
From www.sliderbase.com
Pauling scale of electronegativity; Electronegativity Chlorine Fluorine Its electronegativity value is 3.98. Both of these trends show that fluorine is highly electronegative (it pulls a shared pair of bonding electrons towards itself). Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. It can also be used to predict if the resulting molecule will be polar. Fluorine (the most electronegative. Electronegativity Chlorine Fluorine.
From www.pinterest.com
Electronegativity Definition and Trend Electronegativity Chlorine Fluorine Fluorine is the most electronegative element on the periodic table. Fluorine (the most electronegative element) is assigned a value of 4.0, and values range down to caesium and francium which are the least. Its electronegativity value is 3.98. It can also be used to predict if the resulting molecule will be polar. As you go down a group, electronegativity decreases. Electronegativity Chlorine Fluorine.