Lead 4 Electrons Lost . They form cations by losing electrons, resulting in positively charged ions. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. On the other hand, when a lead atom loses four. Writing formulae of ionic compounds. Why can't lead gain four electrons? Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. Learn about and revise equations and chemical reactions with this bbc bitesize gcse chemistry (ocr 21c) study guide. Metal atoms lose electrons from their outer shell when they form ions: This electron configuration shows that the lead ion(pb 2+) has six shells and the last shell has a total of two electrons. For elements in groups 1, 2 and 3, the number of electrons lost matches the group number. The ions are positive, because they have more protons close proton.
from valenceelectrons.com
On the other hand, when a lead atom loses four. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. For elements in groups 1, 2 and 3, the number of electrons lost matches the group number. The ions are positive, because they have more protons close proton. Metal atoms lose electrons from their outer shell when they form ions: They form cations by losing electrons, resulting in positively charged ions. Writing formulae of ionic compounds. This electron configuration shows that the lead ion(pb 2+) has six shells and the last shell has a total of two electrons. Learn about and revise equations and chemical reactions with this bbc bitesize gcse chemistry (ocr 21c) study guide.
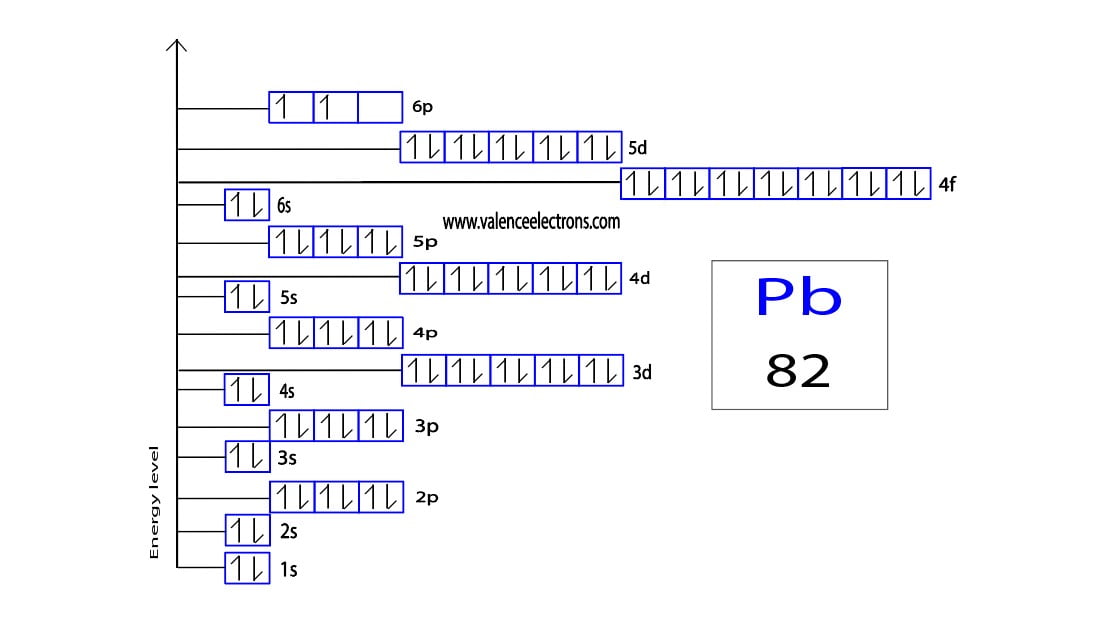
Lead(Pb) Electron Configuration and Orbital Diagram
Lead 4 Electrons Lost They form cations by losing electrons, resulting in positively charged ions. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. This electron configuration shows that the lead ion(pb 2+) has six shells and the last shell has a total of two electrons. Metal atoms lose electrons from their outer shell when they form ions: For elements in groups 1, 2 and 3, the number of electrons lost matches the group number. Learn about and revise equations and chemical reactions with this bbc bitesize gcse chemistry (ocr 21c) study guide. The ions are positive, because they have more protons close proton. Writing formulae of ionic compounds. Why can't lead gain four electrons? Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. On the other hand, when a lead atom loses four. They form cations by losing electrons, resulting in positively charged ions.
From www.slideserve.com
PPT Chapter 22 Chemical Bonds PowerPoint Presentation, free Lead 4 Electrons Lost Learn about and revise equations and chemical reactions with this bbc bitesize gcse chemistry (ocr 21c) study guide. The ions are positive, because they have more protons close proton. Why can't lead gain four electrons? They form cations by losing electrons, resulting in positively charged ions. 93 rows you may assume the valences of the chemical elements—the number of electrons. Lead 4 Electrons Lost.
From www.youtube.com
How to find Protons & Electrons for the Pb2+ and Pb4+ (Lead II and Lead Lead 4 Electrons Lost Learn about and revise equations and chemical reactions with this bbc bitesize gcse chemistry (ocr 21c) study guide. Metal atoms lose electrons from their outer shell when they form ions: They form cations by losing electrons, resulting in positively charged ions. Writing formulae of ionic compounds. 93 rows you may assume the valences of the chemical elements—the number of electrons. Lead 4 Electrons Lost.
From www.slideshare.net
Understanding The Chemistry Of Atoms to Ions Lead 4 Electrons Lost The ions are positive, because they have more protons close proton. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Writing formulae of ionic compounds. Metal atoms lose electrons from their outer shell when they form ions: Atoms tend to lose, gain, or share some valance electrons, making. Lead 4 Electrons Lost.
From www.technocrazed.com
Disposition of electrons entering base (a) Lost due to Lead 4 Electrons Lost The ions are positive, because they have more protons close proton. Writing formulae of ionic compounds. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. This electron configuration shows that the lead ion(pb 2+) has six shells and the last shell has a total of two. Lead 4 Electrons Lost.
From www.slideserve.com
PPT IV. Chemical Bonding PowerPoint Presentation, free download ID Lead 4 Electrons Lost On the other hand, when a lead atom loses four. This electron configuration shows that the lead ion(pb 2+) has six shells and the last shell has a total of two electrons. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. Learn about and revise equations. Lead 4 Electrons Lost.
From www.alamy.com
Atom symbol and electron of lead illustration Stock Vector Art Lead 4 Electrons Lost 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. For elements in groups 1, 2 and 3, the number of electrons lost matches the group number. Metal atoms lose electrons from their outer shell when they form ions: Why can't lead gain four electrons? Learn about and revise. Lead 4 Electrons Lost.
From www.slideserve.com
PPT Chemistry 120 PowerPoint Presentation, free download ID2089971 Lead 4 Electrons Lost For elements in groups 1, 2 and 3, the number of electrons lost matches the group number. They form cations by losing electrons, resulting in positively charged ions. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Learn about and revise equations and chemical reactions with this bbc. Lead 4 Electrons Lost.
From slideplayer.com
Ch. 7 LT1 Ionic Bonding and Metals ppt download Lead 4 Electrons Lost 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Metal atoms lose electrons from their outer shell when they form ions: They form cations by losing electrons, resulting in positively charged ions. For elements in groups 1, 2 and 3, the number of electrons lost matches the group. Lead 4 Electrons Lost.
From www.schoolmykids.com
Lead (Pb) Element Information, Facts, Properties, Uses Periodic Lead 4 Electrons Lost Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. This electron configuration shows that the lead ion(pb 2+) has six shells and the last shell has a total of two electrons. Metal atoms lose electrons from their outer shell when they form ions: On the other. Lead 4 Electrons Lost.
From valenceelectrons.com
Complete Electron Configuration for Lead (Pb, Pb2+, Pb4+) Lead 4 Electrons Lost They form cations by losing electrons, resulting in positively charged ions. For elements in groups 1, 2 and 3, the number of electrons lost matches the group number. Why can't lead gain four electrons? Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. 93 rows you. Lead 4 Electrons Lost.
From www.youtube.com
4.7 Ions Losing & Gaining Electrons YouTube Lead 4 Electrons Lost They form cations by losing electrons, resulting in positively charged ions. Writing formulae of ionic compounds. Why can't lead gain four electrons? Metal atoms lose electrons from their outer shell when they form ions: For elements in groups 1, 2 and 3, the number of electrons lost matches the group number. On the other hand, when a lead atom loses. Lead 4 Electrons Lost.
From exofobtvx.blob.core.windows.net
Does Lead Lose Electrons at Howard Krause blog Lead 4 Electrons Lost This electron configuration shows that the lead ion(pb 2+) has six shells and the last shell has a total of two electrons. They form cations by losing electrons, resulting in positively charged ions. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. On the other hand, when a. Lead 4 Electrons Lost.
From www.researchgate.net
3. Fractional energy loss per radiation length in lead as a function Lead 4 Electrons Lost Why can't lead gain four electrons? Writing formulae of ionic compounds. On the other hand, when a lead atom loses four. The ions are positive, because they have more protons close proton. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. For elements in groups 1, 2 and. Lead 4 Electrons Lost.
From slideplayer.com
Ions and Ionic Compounds ppt download Lead 4 Electrons Lost Metal atoms lose electrons from their outer shell when they form ions: This electron configuration shows that the lead ion(pb 2+) has six shells and the last shell has a total of two electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Writing formulae of ionic compounds.. Lead 4 Electrons Lost.
From www.britannica.com
Lead Definition, Uses, Properties, & Facts Britannica Lead 4 Electrons Lost On the other hand, when a lead atom loses four. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. They form cations by losing electrons, resulting in positively charged ions. Metal atoms lose electrons from their outer shell when they form ions: Learn about and revise. Lead 4 Electrons Lost.
From cabinet.matttroy.net
Lead Periodic Table Protons Neutrons And Electrons Matttroy Lead 4 Electrons Lost Writing formulae of ionic compounds. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. On the other hand, when a lead atom loses four. Metal atoms lose electrons from their outer shell when they form ions: This electron configuration shows that the lead ion(pb 2+) has six shells. Lead 4 Electrons Lost.
From www.youtube.com
Which element is most likely to lose four electrons to form an ionic Lead 4 Electrons Lost Writing formulae of ionic compounds. For elements in groups 1, 2 and 3, the number of electrons lost matches the group number. They form cations by losing electrons, resulting in positively charged ions. This electron configuration shows that the lead ion(pb 2+) has six shells and the last shell has a total of two electrons. Metal atoms lose electrons from. Lead 4 Electrons Lost.
From slideplayer.com
II. Ionic Bonds Part ppt download Lead 4 Electrons Lost On the other hand, when a lead atom loses four. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. This electron configuration shows that the lead ion(pb 2+) has six shells and the last shell has a total of two electrons. Writing formulae of ionic compounds.. Lead 4 Electrons Lost.
From www.youtube.com
Which elements tend to lose electrons? What charge will they Lead 4 Electrons Lost For elements in groups 1, 2 and 3, the number of electrons lost matches the group number. Writing formulae of ionic compounds. Learn about and revise equations and chemical reactions with this bbc bitesize gcse chemistry (ocr 21c) study guide. Why can't lead gain four electrons? Metal atoms lose electrons from their outer shell when they form ions: Atoms tend. Lead 4 Electrons Lost.
From spmchemistry.blog.onlinetuition.com.my
Formation of Ion SPM Chemistry Lead 4 Electrons Lost Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. Writing formulae of ionic compounds. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. On the other hand, when a lead atom loses four. For. Lead 4 Electrons Lost.
From www.mooramo.com
Gaining and Losing Electrons Mooramo Lead 4 Electrons Lost Learn about and revise equations and chemical reactions with this bbc bitesize gcse chemistry (ocr 21c) study guide. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Why can't lead gain four electrons? For elements in groups 1, 2 and 3, the number of electrons lost matches the. Lead 4 Electrons Lost.
From www.bartleby.com
Answered Lead is normally obtained from PbS… bartleby Lead 4 Electrons Lost This electron configuration shows that the lead ion(pb 2+) has six shells and the last shell has a total of two electrons. On the other hand, when a lead atom loses four. Metal atoms lose electrons from their outer shell when they form ions: The ions are positive, because they have more protons close proton. For elements in groups 1,. Lead 4 Electrons Lost.
From ar.inspiredpencil.com
Lead Atom Electrons Lead 4 Electrons Lost Learn about and revise equations and chemical reactions with this bbc bitesize gcse chemistry (ocr 21c) study guide. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. Metal atoms lose electrons from their outer shell when they form ions: They form cations by losing electrons, resulting. Lead 4 Electrons Lost.
From cabinet.matttroy.net
Lead Periodic Table Protons Neutrons And Electrons Matttroy Lead 4 Electrons Lost Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. On the other hand, when a lead atom loses four. Writing formulae of ionic compounds. The ions are positive, because they have more protons close proton. They form cations by losing electrons, resulting in positively charged ions.. Lead 4 Electrons Lost.
From www.youtube.com
CHEMISTRY 101 Writing an Electron Configuration for Lead Using the Lead 4 Electrons Lost On the other hand, when a lead atom loses four. Metal atoms lose electrons from their outer shell when they form ions: Learn about and revise equations and chemical reactions with this bbc bitesize gcse chemistry (ocr 21c) study guide. Writing formulae of ionic compounds. Why can't lead gain four electrons? The ions are positive, because they have more protons. Lead 4 Electrons Lost.
From exofobtvx.blob.core.windows.net
Does Lead Lose Electrons at Howard Krause blog Lead 4 Electrons Lost They form cations by losing electrons, resulting in positively charged ions. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. On the other hand, when. Lead 4 Electrons Lost.
From www.slideserve.com
PPT Ionic and Covalent Bonding PowerPoint Presentation, free download Lead 4 Electrons Lost This electron configuration shows that the lead ion(pb 2+) has six shells and the last shell has a total of two electrons. On the other hand, when a lead atom loses four. The ions are positive, because they have more protons close proton. Metal atoms lose electrons from their outer shell when they form ions: Why can't lead gain four. Lead 4 Electrons Lost.
From exofobtvx.blob.core.windows.net
Does Lead Lose Electrons at Howard Krause blog Lead 4 Electrons Lost For elements in groups 1, 2 and 3, the number of electrons lost matches the group number. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. They form cations by losing electrons, resulting in positively charged ions. Why can't lead gain four electrons? Writing formulae of. Lead 4 Electrons Lost.
From exofobtvx.blob.core.windows.net
Does Lead Lose Electrons at Howard Krause blog Lead 4 Electrons Lost Metal atoms lose electrons from their outer shell when they form ions: On the other hand, when a lead atom loses four. They form cations by losing electrons, resulting in positively charged ions. Writing formulae of ionic compounds. Why can't lead gain four electrons? Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron. Lead 4 Electrons Lost.
From periodictable.me
Lead Valence Electrons Lead Valency (Pb) with Dot Diagram Lead 4 Electrons Lost Why can't lead gain four electrons? This electron configuration shows that the lead ion(pb 2+) has six shells and the last shell has a total of two electrons. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. For elements in groups 1, 2 and 3, the. Lead 4 Electrons Lost.
From valenceelectrons.com
Lead(Pb) Electron Configuration and Orbital Diagram Lead 4 Electrons Lost The ions are positive, because they have more protons close proton. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Writing formulae of ionic compounds. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,.. Lead 4 Electrons Lost.
From ar.inspiredpencil.com
Lead Atom Electrons Lead 4 Electrons Lost Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. Learn about and revise equations and chemical reactions with this bbc bitesize gcse chemistry (ocr 21c) study guide. Metal atoms lose electrons from their outer shell when they form ions: They form cations by losing electrons, resulting. Lead 4 Electrons Lost.
From brainly.com
⚗️5. What is the charge of an atom that has lost four electrons Lead 4 Electrons Lost They form cations by losing electrons, resulting in positively charged ions. The ions are positive, because they have more protons close proton. Writing formulae of ionic compounds. Why can't lead gain four electrons? This electron configuration shows that the lead ion(pb 2+) has six shells and the last shell has a total of two electrons. Metal atoms lose electrons from. Lead 4 Electrons Lost.
From ar.inspiredpencil.com
Lead Atom Electrons Lead 4 Electrons Lost Learn about and revise equations and chemical reactions with this bbc bitesize gcse chemistry (ocr 21c) study guide. Writing formulae of ionic compounds. Metal atoms lose electrons from their outer shell when they form ions: Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. On the. Lead 4 Electrons Lost.
From www.youtube.com
Electron Configuration for Pb, Pb2+, and Pb4+ (Lead and Lead Ions Lead 4 Electrons Lost On the other hand, when a lead atom loses four. Why can't lead gain four electrons? This electron configuration shows that the lead ion(pb 2+) has six shells and the last shell has a total of two electrons. For elements in groups 1, 2 and 3, the number of electrons lost matches the group number. Atoms tend to lose, gain,. Lead 4 Electrons Lost.