Fluorescence Spectroscopy For Tryptophan . In terms of wavelength and intensity, tryptophan fluorescence is strongly influenced by its (or the proteinlocal environment, which, in addition to fluorescence quenching, has been applied to study protein conformational changes. Intrinsic tryptophan fluorescence spectroscopy is an important tool for examining the effects of molecular crowding and confinement on the structure, dynamics, and function of. In this article, we systematically describe the most common applications in fluorescence spectroscopy of proteins, i.e., how to gain. Fluorescence spectroscopy is well suited to obtain information about the structure and function of proteins. If the tryptophan residue of a protein is involved in interaction of the protein with a binding ligand, any change of the. This review aims to provide insight into the utilization of tryptophan (trp). We have predicted the fluorescence wavelengths of 19 tryptophans in 16 proteins, starting with crystal structures and using a.
from www.frontiersin.org
We have predicted the fluorescence wavelengths of 19 tryptophans in 16 proteins, starting with crystal structures and using a. If the tryptophan residue of a protein is involved in interaction of the protein with a binding ligand, any change of the. Fluorescence spectroscopy is well suited to obtain information about the structure and function of proteins. Intrinsic tryptophan fluorescence spectroscopy is an important tool for examining the effects of molecular crowding and confinement on the structure, dynamics, and function of. This review aims to provide insight into the utilization of tryptophan (trp). In this article, we systematically describe the most common applications in fluorescence spectroscopy of proteins, i.e., how to gain. In terms of wavelength and intensity, tryptophan fluorescence is strongly influenced by its (or the proteinlocal environment, which, in addition to fluorescence quenching, has been applied to study protein conformational changes.
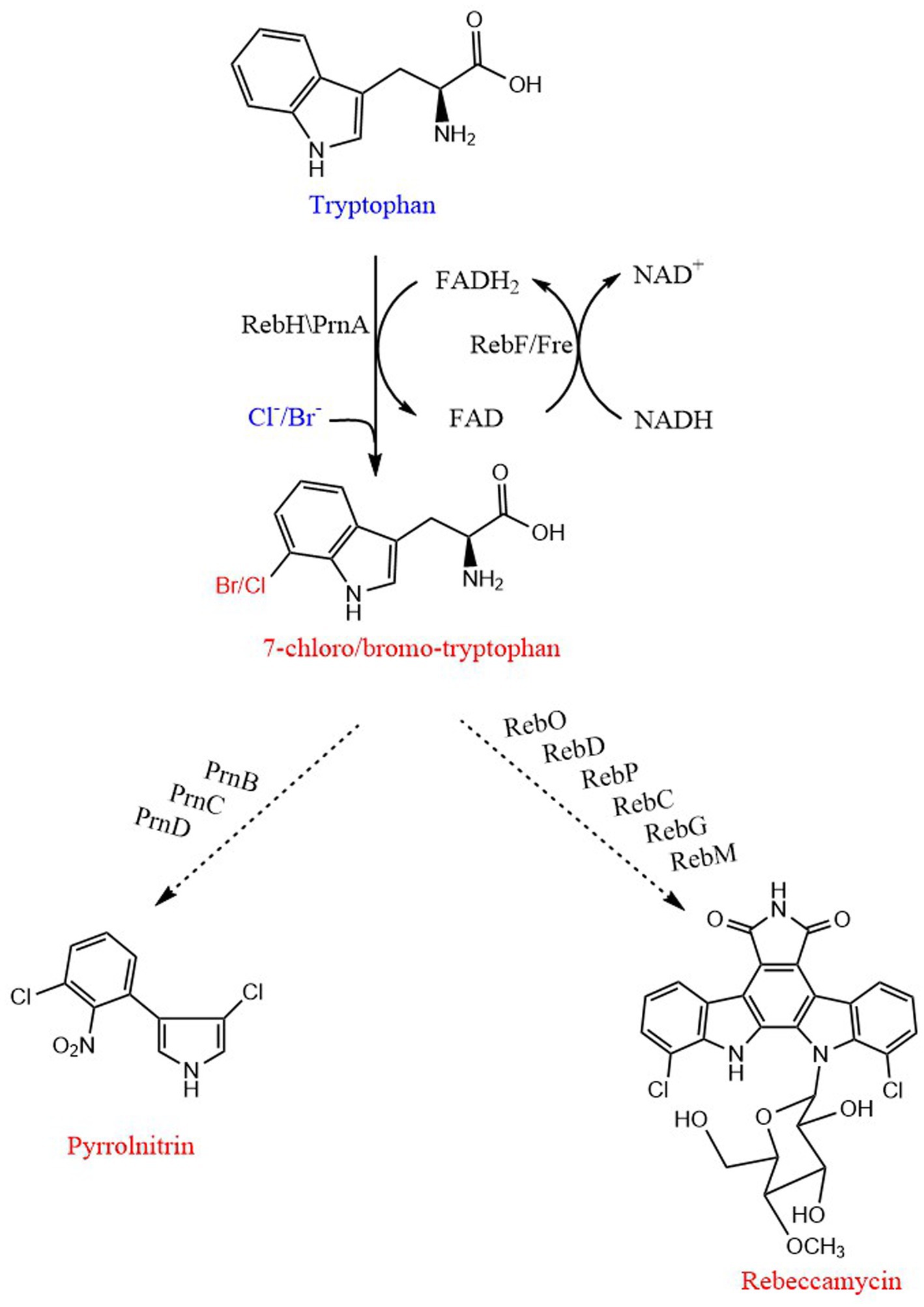
Frontiers Expanding the application of tryptophan Industrial
Fluorescence Spectroscopy For Tryptophan In this article, we systematically describe the most common applications in fluorescence spectroscopy of proteins, i.e., how to gain. This review aims to provide insight into the utilization of tryptophan (trp). In this article, we systematically describe the most common applications in fluorescence spectroscopy of proteins, i.e., how to gain. Intrinsic tryptophan fluorescence spectroscopy is an important tool for examining the effects of molecular crowding and confinement on the structure, dynamics, and function of. If the tryptophan residue of a protein is involved in interaction of the protein with a binding ligand, any change of the. In terms of wavelength and intensity, tryptophan fluorescence is strongly influenced by its (or the proteinlocal environment, which, in addition to fluorescence quenching, has been applied to study protein conformational changes. Fluorescence spectroscopy is well suited to obtain information about the structure and function of proteins. We have predicted the fluorescence wavelengths of 19 tryptophans in 16 proteins, starting with crystal structures and using a.
From www.myxxgirl.com
Bacterial Map Of Tryptophan Metabolism My XXX Hot Girl Fluorescence Spectroscopy For Tryptophan Fluorescence spectroscopy is well suited to obtain information about the structure and function of proteins. This review aims to provide insight into the utilization of tryptophan (trp). If the tryptophan residue of a protein is involved in interaction of the protein with a binding ligand, any change of the. We have predicted the fluorescence wavelengths of 19 tryptophans in 16. Fluorescence Spectroscopy For Tryptophan.
From www.researchgate.net
Representative fluorescent emission spectra of tryptophan. The Fluorescence Spectroscopy For Tryptophan In terms of wavelength and intensity, tryptophan fluorescence is strongly influenced by its (or the proteinlocal environment, which, in addition to fluorescence quenching, has been applied to study protein conformational changes. In this article, we systematically describe the most common applications in fluorescence spectroscopy of proteins, i.e., how to gain. If the tryptophan residue of a protein is involved in. Fluorescence Spectroscopy For Tryptophan.
From www.mdpi.com
Algorithms Free FullText Algorithm for the Analysis of Tryptophan Fluorescence Spectroscopy For Tryptophan In terms of wavelength and intensity, tryptophan fluorescence is strongly influenced by its (or the proteinlocal environment, which, in addition to fluorescence quenching, has been applied to study protein conformational changes. If the tryptophan residue of a protein is involved in interaction of the protein with a binding ligand, any change of the. Fluorescence spectroscopy is well suited to obtain. Fluorescence Spectroscopy For Tryptophan.
From www2.mdpi.com
Molecules Free FullText LCMS/MSBased Profiling of Tryptophan Fluorescence Spectroscopy For Tryptophan Fluorescence spectroscopy is well suited to obtain information about the structure and function of proteins. Intrinsic tryptophan fluorescence spectroscopy is an important tool for examining the effects of molecular crowding and confinement on the structure, dynamics, and function of. In this article, we systematically describe the most common applications in fluorescence spectroscopy of proteins, i.e., how to gain. In terms. Fluorescence Spectroscopy For Tryptophan.
From www.nature.com
Functional dynamics of a single tryptophan residue in a BLUF protein Fluorescence Spectroscopy For Tryptophan Fluorescence spectroscopy is well suited to obtain information about the structure and function of proteins. Intrinsic tryptophan fluorescence spectroscopy is an important tool for examining the effects of molecular crowding and confinement on the structure, dynamics, and function of. In terms of wavelength and intensity, tryptophan fluorescence is strongly influenced by its (or the proteinlocal environment, which, in addition to. Fluorescence Spectroscopy For Tryptophan.
From www.mdpi.com
Free FullText MicrobialDerived Tryptophan Fluorescence Spectroscopy For Tryptophan This review aims to provide insight into the utilization of tryptophan (trp). Intrinsic tryptophan fluorescence spectroscopy is an important tool for examining the effects of molecular crowding and confinement on the structure, dynamics, and function of. In this article, we systematically describe the most common applications in fluorescence spectroscopy of proteins, i.e., how to gain. We have predicted the fluorescence. Fluorescence Spectroscopy For Tryptophan.
From www.dreamstime.com
Tryptophan Chemical Formula. Tryptophan Chemical Molecular Structure Fluorescence Spectroscopy For Tryptophan Intrinsic tryptophan fluorescence spectroscopy is an important tool for examining the effects of molecular crowding and confinement on the structure, dynamics, and function of. In this article, we systematically describe the most common applications in fluorescence spectroscopy of proteins, i.e., how to gain. We have predicted the fluorescence wavelengths of 19 tryptophans in 16 proteins, starting with crystal structures and. Fluorescence Spectroscopy For Tryptophan.
From cartoondealer.com
Tryptophan Or Ltryptophan, Trp, W Amino Acid Molecule. Skeletal Fluorescence Spectroscopy For Tryptophan This review aims to provide insight into the utilization of tryptophan (trp). Intrinsic tryptophan fluorescence spectroscopy is an important tool for examining the effects of molecular crowding and confinement on the structure, dynamics, and function of. Fluorescence spectroscopy is well suited to obtain information about the structure and function of proteins. If the tryptophan residue of a protein is involved. Fluorescence Spectroscopy For Tryptophan.
From www.researchgate.net
(a) Tryptophan fluorescence spectroscopy of the hPSA epitope RHSLFHP Fluorescence Spectroscopy For Tryptophan In this article, we systematically describe the most common applications in fluorescence spectroscopy of proteins, i.e., how to gain. In terms of wavelength and intensity, tryptophan fluorescence is strongly influenced by its (or the proteinlocal environment, which, in addition to fluorescence quenching, has been applied to study protein conformational changes. If the tryptophan residue of a protein is involved in. Fluorescence Spectroscopy For Tryptophan.
From www.coursehero.com
[Solved] Starting with the precursor substance tyrosine or tryptophan Fluorescence Spectroscopy For Tryptophan This review aims to provide insight into the utilization of tryptophan (trp). If the tryptophan residue of a protein is involved in interaction of the protein with a binding ligand, any change of the. In this article, we systematically describe the most common applications in fluorescence spectroscopy of proteins, i.e., how to gain. In terms of wavelength and intensity, tryptophan. Fluorescence Spectroscopy For Tryptophan.
From www.researchgate.net
(A) Using tryptophan fluorescence spectroscopy MetQ (0.05 µM) in 50 mM Fluorescence Spectroscopy For Tryptophan In this article, we systematically describe the most common applications in fluorescence spectroscopy of proteins, i.e., how to gain. In terms of wavelength and intensity, tryptophan fluorescence is strongly influenced by its (or the proteinlocal environment, which, in addition to fluorescence quenching, has been applied to study protein conformational changes. If the tryptophan residue of a protein is involved in. Fluorescence Spectroscopy For Tryptophan.
From www.researchgate.net
(a) Fluorescence spectrum (λ l ext =290 nm) of tryptophan of HSA (20 Fluorescence Spectroscopy For Tryptophan Intrinsic tryptophan fluorescence spectroscopy is an important tool for examining the effects of molecular crowding and confinement on the structure, dynamics, and function of. This review aims to provide insight into the utilization of tryptophan (trp). If the tryptophan residue of a protein is involved in interaction of the protein with a binding ligand, any change of the. Fluorescence spectroscopy. Fluorescence Spectroscopy For Tryptophan.
From www.dreamstime.com
Tryptophan Amino Acid. Chemical Molecular Formula of Tryptophan Amino Fluorescence Spectroscopy For Tryptophan If the tryptophan residue of a protein is involved in interaction of the protein with a binding ligand, any change of the. Intrinsic tryptophan fluorescence spectroscopy is an important tool for examining the effects of molecular crowding and confinement on the structure, dynamics, and function of. This review aims to provide insight into the utilization of tryptophan (trp). In terms. Fluorescence Spectroscopy For Tryptophan.
From www.mdpi.com
Molecules Free FullText Design, Synthesis, and Bioactivities of Fluorescence Spectroscopy For Tryptophan We have predicted the fluorescence wavelengths of 19 tryptophans in 16 proteins, starting with crystal structures and using a. In terms of wavelength and intensity, tryptophan fluorescence is strongly influenced by its (or the proteinlocal environment, which, in addition to fluorescence quenching, has been applied to study protein conformational changes. Fluorescence spectroscopy is well suited to obtain information about the. Fluorescence Spectroscopy For Tryptophan.
From cartoondealer.com
LTryptophan Structural Formula On Blue Medical Background With Fluorescence Spectroscopy For Tryptophan Intrinsic tryptophan fluorescence spectroscopy is an important tool for examining the effects of molecular crowding and confinement on the structure, dynamics, and function of. We have predicted the fluorescence wavelengths of 19 tryptophans in 16 proteins, starting with crystal structures and using a. If the tryptophan residue of a protein is involved in interaction of the protein with a binding. Fluorescence Spectroscopy For Tryptophan.
From www.mdpi.com
Antioxidants Free FullText Kynurenine Pathway of Tryptophan Fluorescence Spectroscopy For Tryptophan Fluorescence spectroscopy is well suited to obtain information about the structure and function of proteins. We have predicted the fluorescence wavelengths of 19 tryptophans in 16 proteins, starting with crystal structures and using a. If the tryptophan residue of a protein is involved in interaction of the protein with a binding ligand, any change of the. Intrinsic tryptophan fluorescence spectroscopy. Fluorescence Spectroscopy For Tryptophan.
From www.frontiersin.org
Frontiers Immunomodulatory Effects of Tryptophan Metabolism in the Fluorescence Spectroscopy For Tryptophan Fluorescence spectroscopy is well suited to obtain information about the structure and function of proteins. Intrinsic tryptophan fluorescence spectroscopy is an important tool for examining the effects of molecular crowding and confinement on the structure, dynamics, and function of. In this article, we systematically describe the most common applications in fluorescence spectroscopy of proteins, i.e., how to gain. If the. Fluorescence Spectroscopy For Tryptophan.
From cartoondealer.com
LTryptophan Structural Formula On Blue Medical Background With Fluorescence Spectroscopy For Tryptophan In this article, we systematically describe the most common applications in fluorescence spectroscopy of proteins, i.e., how to gain. In terms of wavelength and intensity, tryptophan fluorescence is strongly influenced by its (or the proteinlocal environment, which, in addition to fluorescence quenching, has been applied to study protein conformational changes. This review aims to provide insight into the utilization of. Fluorescence Spectroscopy For Tryptophan.
From www.mdpi.com
IJMS Free FullText Sex Differences in Tryptophan Metabolism A Fluorescence Spectroscopy For Tryptophan In terms of wavelength and intensity, tryptophan fluorescence is strongly influenced by its (or the proteinlocal environment, which, in addition to fluorescence quenching, has been applied to study protein conformational changes. If the tryptophan residue of a protein is involved in interaction of the protein with a binding ligand, any change of the. Fluorescence spectroscopy is well suited to obtain. Fluorescence Spectroscopy For Tryptophan.
From www.frontiersin.org
Frontiers Expanding the application of tryptophan Industrial Fluorescence Spectroscopy For Tryptophan In terms of wavelength and intensity, tryptophan fluorescence is strongly influenced by its (or the proteinlocal environment, which, in addition to fluorescence quenching, has been applied to study protein conformational changes. This review aims to provide insight into the utilization of tryptophan (trp). Fluorescence spectroscopy is well suited to obtain information about the structure and function of proteins. If the. Fluorescence Spectroscopy For Tryptophan.
From www.mdpi.com
IJMS Free FullText Intrinsic Tryptophan Fluorescence in the Fluorescence Spectroscopy For Tryptophan We have predicted the fluorescence wavelengths of 19 tryptophans in 16 proteins, starting with crystal structures and using a. This review aims to provide insight into the utilization of tryptophan (trp). Fluorescence spectroscopy is well suited to obtain information about the structure and function of proteins. If the tryptophan residue of a protein is involved in interaction of the protein. Fluorescence Spectroscopy For Tryptophan.
From www2.mdpi.com
IJMS Free FullText Tryptophan Metabolism and GutBrain Homeostasis Fluorescence Spectroscopy For Tryptophan In this article, we systematically describe the most common applications in fluorescence spectroscopy of proteins, i.e., how to gain. Fluorescence spectroscopy is well suited to obtain information about the structure and function of proteins. We have predicted the fluorescence wavelengths of 19 tryptophans in 16 proteins, starting with crystal structures and using a. In terms of wavelength and intensity, tryptophan. Fluorescence Spectroscopy For Tryptophan.
From www.mdpi.com
IJMS Free FullText The Tryptophan and Kynurenine Pathway Involved Fluorescence Spectroscopy For Tryptophan Intrinsic tryptophan fluorescence spectroscopy is an important tool for examining the effects of molecular crowding and confinement on the structure, dynamics, and function of. This review aims to provide insight into the utilization of tryptophan (trp). In this article, we systematically describe the most common applications in fluorescence spectroscopy of proteins, i.e., how to gain. In terms of wavelength and. Fluorescence Spectroscopy For Tryptophan.
From www.frontiersin.org
Frontiers Expanding the application of tryptophan Industrial Fluorescence Spectroscopy For Tryptophan In this article, we systematically describe the most common applications in fluorescence spectroscopy of proteins, i.e., how to gain. This review aims to provide insight into the utilization of tryptophan (trp). We have predicted the fluorescence wavelengths of 19 tryptophans in 16 proteins, starting with crystal structures and using a. Intrinsic tryptophan fluorescence spectroscopy is an important tool for examining. Fluorescence Spectroscopy For Tryptophan.
From www.mdpi.com
IJMS Free FullText The Tryptophan and Kynurenine Pathway Involved Fluorescence Spectroscopy For Tryptophan Fluorescence spectroscopy is well suited to obtain information about the structure and function of proteins. We have predicted the fluorescence wavelengths of 19 tryptophans in 16 proteins, starting with crystal structures and using a. Intrinsic tryptophan fluorescence spectroscopy is an important tool for examining the effects of molecular crowding and confinement on the structure, dynamics, and function of. In this. Fluorescence Spectroscopy For Tryptophan.
From vajiramias.com
Researchers have recently discovered the amino acid tryptophan in Fluorescence Spectroscopy For Tryptophan We have predicted the fluorescence wavelengths of 19 tryptophans in 16 proteins, starting with crystal structures and using a. Fluorescence spectroscopy is well suited to obtain information about the structure and function of proteins. In this article, we systematically describe the most common applications in fluorescence spectroscopy of proteins, i.e., how to gain. In terms of wavelength and intensity, tryptophan. Fluorescence Spectroscopy For Tryptophan.
From www.researchgate.net
Tryptophan fluorescence spectra of BPMV components. The top component Fluorescence Spectroscopy For Tryptophan In terms of wavelength and intensity, tryptophan fluorescence is strongly influenced by its (or the proteinlocal environment, which, in addition to fluorescence quenching, has been applied to study protein conformational changes. Fluorescence spectroscopy is well suited to obtain information about the structure and function of proteins. This review aims to provide insight into the utilization of tryptophan (trp). We have. Fluorescence Spectroscopy For Tryptophan.
From www.dreamstime.com
Tryptophan, Trp or W Amino Acid Molecule, is Used in the Biosynthesis Fluorescence Spectroscopy For Tryptophan In terms of wavelength and intensity, tryptophan fluorescence is strongly influenced by its (or the proteinlocal environment, which, in addition to fluorescence quenching, has been applied to study protein conformational changes. This review aims to provide insight into the utilization of tryptophan (trp). Fluorescence spectroscopy is well suited to obtain information about the structure and function of proteins. Intrinsic tryptophan. Fluorescence Spectroscopy For Tryptophan.
From www.dreamstime.com
Tryptophan Chemical Structure. Vector Illustration Hand Drawn. Stock Fluorescence Spectroscopy For Tryptophan In this article, we systematically describe the most common applications in fluorescence spectroscopy of proteins, i.e., how to gain. If the tryptophan residue of a protein is involved in interaction of the protein with a binding ligand, any change of the. Fluorescence spectroscopy is well suited to obtain information about the structure and function of proteins. In terms of wavelength. Fluorescence Spectroscopy For Tryptophan.
From www.researchgate.net
a Representative fluorescence emission spectrum of tryptophan residues Fluorescence Spectroscopy For Tryptophan In terms of wavelength and intensity, tryptophan fluorescence is strongly influenced by its (or the proteinlocal environment, which, in addition to fluorescence quenching, has been applied to study protein conformational changes. If the tryptophan residue of a protein is involved in interaction of the protein with a binding ligand, any change of the. This review aims to provide insight into. Fluorescence Spectroscopy For Tryptophan.
From www.researchgate.net
Tryptophan fluorescence spectroscopy of purified His 6 LivJ Download Fluorescence Spectroscopy For Tryptophan Intrinsic tryptophan fluorescence spectroscopy is an important tool for examining the effects of molecular crowding and confinement on the structure, dynamics, and function of. In terms of wavelength and intensity, tryptophan fluorescence is strongly influenced by its (or the proteinlocal environment, which, in addition to fluorescence quenching, has been applied to study protein conformational changes. In this article, we systematically. Fluorescence Spectroscopy For Tryptophan.
From www.researchgate.net
Intrinsic tryptophan fluorescence spectra at various pressures and Fluorescence Spectroscopy For Tryptophan This review aims to provide insight into the utilization of tryptophan (trp). If the tryptophan residue of a protein is involved in interaction of the protein with a binding ligand, any change of the. We have predicted the fluorescence wavelengths of 19 tryptophans in 16 proteins, starting with crystal structures and using a. In terms of wavelength and intensity, tryptophan. Fluorescence Spectroscopy For Tryptophan.
From www.researchgate.net
Fluorescence spectra of tyrosine at excitation wavelength of 200 nm (a Fluorescence Spectroscopy For Tryptophan We have predicted the fluorescence wavelengths of 19 tryptophans in 16 proteins, starting with crystal structures and using a. In terms of wavelength and intensity, tryptophan fluorescence is strongly influenced by its (or the proteinlocal environment, which, in addition to fluorescence quenching, has been applied to study protein conformational changes. Fluorescence spectroscopy is well suited to obtain information about the. Fluorescence Spectroscopy For Tryptophan.
From www.alamy.com
Tryptophan trp amino acid molecular Black and White Stock Photos Fluorescence Spectroscopy For Tryptophan We have predicted the fluorescence wavelengths of 19 tryptophans in 16 proteins, starting with crystal structures and using a. In this article, we systematically describe the most common applications in fluorescence spectroscopy of proteins, i.e., how to gain. Fluorescence spectroscopy is well suited to obtain information about the structure and function of proteins. If the tryptophan residue of a protein. Fluorescence Spectroscopy For Tryptophan.
From www.frontiersin.org
Frontiers Expanding the application of tryptophan Industrial Fluorescence Spectroscopy For Tryptophan In this article, we systematically describe the most common applications in fluorescence spectroscopy of proteins, i.e., how to gain. Fluorescence spectroscopy is well suited to obtain information about the structure and function of proteins. This review aims to provide insight into the utilization of tryptophan (trp). If the tryptophan residue of a protein is involved in interaction of the protein. Fluorescence Spectroscopy For Tryptophan.