Sodium Chloride And Lead Ii Nitrate Formula . When sodium chloride is added to the lead nitrate solution a precipitate of lead chloride and sodium nitrate is formed. Enter an equation of an ionic chemical equation and press the balance button. When aqueous lead nitrate (pb (no 3) 2) and aqueous or solid sodium chloride (hcl) are reacted, lead chloride (pbcl 2) precipitate and. The products of the reaction are an aqueous solution of sodium nitrate and a. In this class practical, students react two soluble metal salts to produce one soluble salt and one insoluble salt. Using the procedure below, the experiment should take no more than 30. Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate. Aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. The balanced equation will be calculated along with the. But for the net ionic equation, we represent only the net, macroscopic chemical change:
from www.bartleby.com
The products of the reaction are an aqueous solution of sodium nitrate and a. The balanced equation will be calculated along with the. Aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate. Using the procedure below, the experiment should take no more than 30. Enter an equation of an ionic chemical equation and press the balance button. But for the net ionic equation, we represent only the net, macroscopic chemical change: When sodium chloride is added to the lead nitrate solution a precipitate of lead chloride and sodium nitrate is formed. In this class practical, students react two soluble metal salts to produce one soluble salt and one insoluble salt. When aqueous lead nitrate (pb (no 3) 2) and aqueous or solid sodium chloride (hcl) are reacted, lead chloride (pbcl 2) precipitate and.
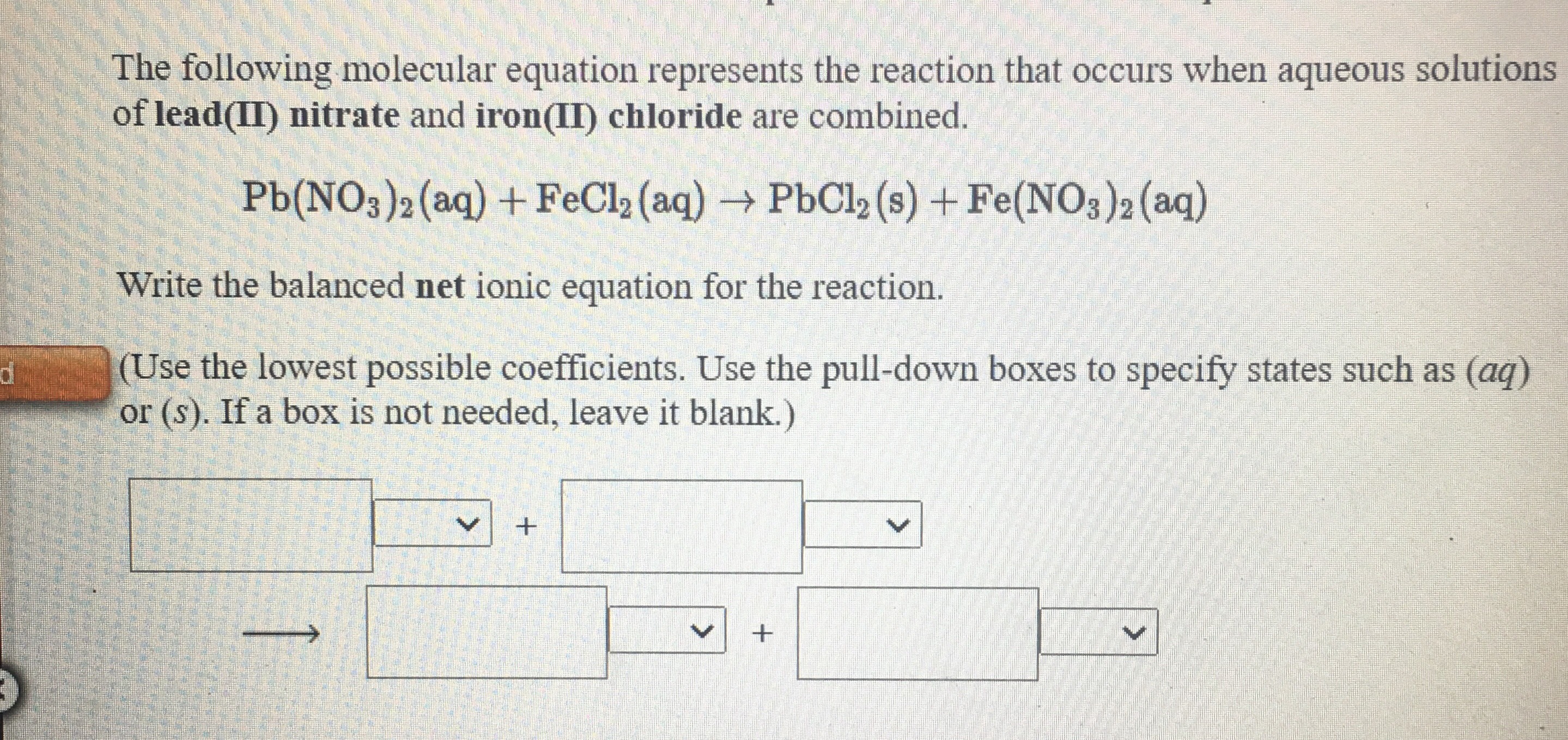
Answered When aqueous solutions of iron(II)… bartleby
Sodium Chloride And Lead Ii Nitrate Formula The products of the reaction are an aqueous solution of sodium nitrate and a. The products of the reaction are an aqueous solution of sodium nitrate and a. Enter an equation of an ionic chemical equation and press the balance button. But for the net ionic equation, we represent only the net, macroscopic chemical change: When aqueous lead nitrate (pb (no 3) 2) and aqueous or solid sodium chloride (hcl) are reacted, lead chloride (pbcl 2) precipitate and. Aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate. When sodium chloride is added to the lead nitrate solution a precipitate of lead chloride and sodium nitrate is formed. The balanced equation will be calculated along with the. Using the procedure below, the experiment should take no more than 30. In this class practical, students react two soluble metal salts to produce one soluble salt and one insoluble salt.
From wisc.pb.unizin.org
Solutions and Solubility (part 2) (M3Q2) UWMadison Chemistry 103/104 Sodium Chloride And Lead Ii Nitrate Formula When sodium chloride is added to the lead nitrate solution a precipitate of lead chloride and sodium nitrate is formed. Enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be calculated along with the. But for the net ionic equation, we represent only the net, macroscopic chemical change: In this class practical,. Sodium Chloride And Lead Ii Nitrate Formula.
From www.numerade.com
SOLVEDDoes a reaction occur when aqueous solutions of sodium sulfate Sodium Chloride And Lead Ii Nitrate Formula Enter an equation of an ionic chemical equation and press the balance button. Aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate. When aqueous lead nitrate (pb (no 3) 2) and aqueous or solid sodium chloride (hcl) are reacted,. Sodium Chloride And Lead Ii Nitrate Formula.
From www.coursehero.com
[Solved] If I start with 25.0 grams of lead (II) nitrate and 15.0 grams Sodium Chloride And Lead Ii Nitrate Formula When aqueous lead nitrate (pb (no 3) 2) and aqueous or solid sodium chloride (hcl) are reacted, lead chloride (pbcl 2) precipitate and. When sodium chloride is added to the lead nitrate solution a precipitate of lead chloride and sodium nitrate is formed. Aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. The balanced equation will be calculated. Sodium Chloride And Lead Ii Nitrate Formula.
From www.slideserve.com
PPT Number One Sodium chloride solution + lead (II) acetate solutions Sodium Chloride And Lead Ii Nitrate Formula When sodium chloride is added to the lead nitrate solution a precipitate of lead chloride and sodium nitrate is formed. In this class practical, students react two soluble metal salts to produce one soluble salt and one insoluble salt. Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate. Using the. Sodium Chloride And Lead Ii Nitrate Formula.
From www.chegg.com
Solved An aqueous solution of lead(II) nitrate is mixed with Sodium Chloride And Lead Ii Nitrate Formula But for the net ionic equation, we represent only the net, macroscopic chemical change: Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate. When sodium chloride is added to the lead nitrate solution a precipitate of lead chloride and sodium nitrate is formed. When aqueous lead nitrate (pb (no 3). Sodium Chloride And Lead Ii Nitrate Formula.
From www.tessshebaylo.com
Balanced Chemical Equation For Sodium Sulfate And Water Tessshebaylo Sodium Chloride And Lead Ii Nitrate Formula Enter an equation of an ionic chemical equation and press the balance button. When sodium chloride is added to the lead nitrate solution a precipitate of lead chloride and sodium nitrate is formed. Using the procedure below, the experiment should take no more than 30. The balanced equation will be calculated along with the. In this class practical, students react. Sodium Chloride And Lead Ii Nitrate Formula.
From www.numerade.com
⏩SOLVEDCopper(Il) chloride and lead(II) nitrate react in aqueous Sodium Chloride And Lead Ii Nitrate Formula Aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. In this class practical, students react two soluble metal salts to produce one soluble salt and one insoluble salt. But for the net ionic equation, we represent only the net, macroscopic chemical change: When sodium chloride is added to the lead nitrate solution a precipitate of lead chloride and. Sodium Chloride And Lead Ii Nitrate Formula.
From www.numerade.com
SOLVED Aqueous ammonium chloride and aqueous lead(II) nitrate reacts Sodium Chloride And Lead Ii Nitrate Formula Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate. The products of the reaction are an aqueous solution of sodium nitrate and a. The balanced equation will be calculated along with the. Using the procedure below, the experiment should take no more than 30. But for the net ionic equation,. Sodium Chloride And Lead Ii Nitrate Formula.
From slideplayer.com
Precipitation Reactions ppt download Sodium Chloride And Lead Ii Nitrate Formula Using the procedure below, the experiment should take no more than 30. The balanced equation will be calculated along with the. Aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. When aqueous lead nitrate (pb (no 3) 2) and aqueous or solid sodium chloride (hcl) are reacted, lead chloride (pbcl 2) precipitate and. Lead(ii) chloride, a white precipitate,. Sodium Chloride And Lead Ii Nitrate Formula.
From www.numerade.com
SOLVEDPostlab Practice 1.0 &.) Write the balanced equation for the Sodium Chloride And Lead Ii Nitrate Formula Using the procedure below, the experiment should take no more than 30. In this class practical, students react two soluble metal salts to produce one soluble salt and one insoluble salt. When sodium chloride is added to the lead nitrate solution a precipitate of lead chloride and sodium nitrate is formed. The products of the reaction are an aqueous solution. Sodium Chloride And Lead Ii Nitrate Formula.
From tiannagroharvey.blogspot.com
Aqueous Sodium Chloride Reacts With Aqueous Lead Ii Nitrate Sodium Chloride And Lead Ii Nitrate Formula The balanced equation will be calculated along with the. When sodium chloride is added to the lead nitrate solution a precipitate of lead chloride and sodium nitrate is formed. Aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. Using the procedure below, the experiment should take no more than 30. In this class practical, students react two soluble. Sodium Chloride And Lead Ii Nitrate Formula.
From www.youtube.com
Double displacement NaCl + Pb(NO3)2 Sodium chloride + Lead(ii Sodium Chloride And Lead Ii Nitrate Formula The balanced equation will be calculated along with the. In this class practical, students react two soluble metal salts to produce one soluble salt and one insoluble salt. Enter an equation of an ionic chemical equation and press the balance button. Aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. Using the procedure below, the experiment should take. Sodium Chloride And Lead Ii Nitrate Formula.
From questions.kunduz.com
Aluminum reacts with lead (II) nitrate a... Chemistry Sodium Chloride And Lead Ii Nitrate Formula In this class practical, students react two soluble metal salts to produce one soluble salt and one insoluble salt. But for the net ionic equation, we represent only the net, macroscopic chemical change: Aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. The balanced equation will be calculated along with the. Using the procedure below, the experiment should. Sodium Chloride And Lead Ii Nitrate Formula.
From testbook.com
Lead (II) Nitrate Formula Structure, Preparation, & Uses Sodium Chloride And Lead Ii Nitrate Formula In this class practical, students react two soluble metal salts to produce one soluble salt and one insoluble salt. Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate. Enter an equation of an ionic chemical equation and press the balance button. But for the net ionic equation, we represent only. Sodium Chloride And Lead Ii Nitrate Formula.
From www.slideserve.com
PPT Reactions in Aqueous Solution PowerPoint Presentation, free Sodium Chloride And Lead Ii Nitrate Formula Aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. The products of the reaction are an aqueous solution of sodium nitrate and a. But for the net ionic equation, we represent only the net, macroscopic chemical change: When sodium chloride is added to the lead nitrate solution a precipitate of lead chloride and sodium nitrate is formed. Enter. Sodium Chloride And Lead Ii Nitrate Formula.
From www.coursehero.com
[Solved] 13_ Aqueous lead (1]) nitrate, Ph[N03)2 undergoes a double Sodium Chloride And Lead Ii Nitrate Formula But for the net ionic equation, we represent only the net, macroscopic chemical change: The balanced equation will be calculated along with the. Aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. In this class practical, students react two soluble metal salts to produce one soluble salt and one insoluble salt. Using the procedure below, the experiment should. Sodium Chloride And Lead Ii Nitrate Formula.
From www.numerade.com
SOLVED Write a balanced equation for the reaction between aqueous lead Sodium Chloride And Lead Ii Nitrate Formula Using the procedure below, the experiment should take no more than 30. Aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. But for the net ionic equation, we represent only the net, macroscopic chemical change: When sodium chloride is added to the lead nitrate solution a precipitate of lead chloride and sodium nitrate is formed. Lead(ii) chloride, a. Sodium Chloride And Lead Ii Nitrate Formula.
From www.coursehero.com
[Solved] Aqueous lead (II) nitrate, Pb(NO3)2 undergoes a double Sodium Chloride And Lead Ii Nitrate Formula Aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. But for the net ionic equation, we represent only the net, macroscopic chemical change: In this class practical, students react two soluble metal salts to produce one soluble salt and one insoluble salt. When sodium chloride is added to the lead nitrate solution a precipitate of lead chloride and. Sodium Chloride And Lead Ii Nitrate Formula.
From www.chegg.com
Solved When sodium chloride and lead (II) nitrate react in Sodium Chloride And Lead Ii Nitrate Formula Aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. Enter an equation of an ionic chemical equation and press the balance button. But for the net ionic equation, we represent only the net, macroscopic chemical change: In this class practical, students react two soluble metal salts to produce one soluble salt and one insoluble salt. Using the procedure. Sodium Chloride And Lead Ii Nitrate Formula.
From hxemtsrvi.blob.core.windows.net
Lead Ii Nitrate Equation at Donald Ramos blog Sodium Chloride And Lead Ii Nitrate Formula Using the procedure below, the experiment should take no more than 30. Aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. Enter an equation of an ionic chemical equation and press the balance button. The products of the reaction are an aqueous solution of sodium nitrate and a. When sodium chloride is added to the lead nitrate solution. Sodium Chloride And Lead Ii Nitrate Formula.
From lab.honeywell.com
Lead(II) nitrate 228621 Honeywell Research Chemicals Sodium Chloride And Lead Ii Nitrate Formula Using the procedure below, the experiment should take no more than 30. The products of the reaction are an aqueous solution of sodium nitrate and a. The balanced equation will be calculated along with the. Enter an equation of an ionic chemical equation and press the balance button. Aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. Lead(ii). Sodium Chloride And Lead Ii Nitrate Formula.
From www.tessshebaylo.com
Chemical Equation For Water And Sodium Chloride Tessshebaylo Sodium Chloride And Lead Ii Nitrate Formula The balanced equation will be calculated along with the. When sodium chloride is added to the lead nitrate solution a precipitate of lead chloride and sodium nitrate is formed. But for the net ionic equation, we represent only the net, macroscopic chemical change: Using the procedure below, the experiment should take no more than 30. Enter an equation of an. Sodium Chloride And Lead Ii Nitrate Formula.
From fphoto.photoshelter.com
science chemistry precipitation reaction lead (ii) chloride Sodium Chloride And Lead Ii Nitrate Formula Aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. Enter an equation of an ionic chemical equation and press the balance button. When sodium chloride is added to the lead nitrate solution a precipitate of lead chloride and sodium nitrate is formed. When aqueous lead nitrate (pb (no 3) 2) and aqueous or solid sodium chloride (hcl) are. Sodium Chloride And Lead Ii Nitrate Formula.
From hxemtsrvi.blob.core.windows.net
Lead Ii Nitrate Equation at Donald Ramos blog Sodium Chloride And Lead Ii Nitrate Formula Enter an equation of an ionic chemical equation and press the balance button. Using the procedure below, the experiment should take no more than 30. Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate. In this class practical, students react two soluble metal salts to produce one soluble salt and. Sodium Chloride And Lead Ii Nitrate Formula.
From www.youtube.com
How to Write the Net Ionic Equation for Na2S + Pb(NO3)2 = NaNO3 + PbS Sodium Chloride And Lead Ii Nitrate Formula Aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. In this class practical, students react two soluble metal salts to produce one soluble salt and one insoluble salt. But for the net ionic equation, we represent only the net, macroscopic chemical change: Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid). Sodium Chloride And Lead Ii Nitrate Formula.
From www.youtube.com
Write the balanced chemical equation for each of the following Sodium Chloride And Lead Ii Nitrate Formula Using the procedure below, the experiment should take no more than 30. In this class practical, students react two soluble metal salts to produce one soluble salt and one insoluble salt. But for the net ionic equation, we represent only the net, macroscopic chemical change: Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric. Sodium Chloride And Lead Ii Nitrate Formula.
From www.chegg.com
Solved If 1 mole of lead (II) nitrate reacts with 1 mole of Sodium Chloride And Lead Ii Nitrate Formula Enter an equation of an ionic chemical equation and press the balance button. Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate. When aqueous lead nitrate (pb (no 3) 2) and aqueous or solid sodium chloride (hcl) are reacted, lead chloride (pbcl 2) precipitate and. Using the procedure below, the. Sodium Chloride And Lead Ii Nitrate Formula.
From www.bartleby.com
Answered When aqueous solutions of iron(II)… bartleby Sodium Chloride And Lead Ii Nitrate Formula The balanced equation will be calculated along with the. Using the procedure below, the experiment should take no more than 30. When sodium chloride is added to the lead nitrate solution a precipitate of lead chloride and sodium nitrate is formed. Aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. Enter an equation of an ionic chemical equation. Sodium Chloride And Lead Ii Nitrate Formula.
From www.slideserve.com
PPT Reactions Involving Ions Molecular vs. Ionic Equations Sodium Chloride And Lead Ii Nitrate Formula In this class practical, students react two soluble metal salts to produce one soluble salt and one insoluble salt. Aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. But for the net ionic equation, we represent only the net, macroscopic chemical change: The balanced equation will be calculated along with the. Using the procedure below, the experiment should. Sodium Chloride And Lead Ii Nitrate Formula.
From www.numerade.com
SOLVED Aqueous lead (II) nitrate, Pb(NO3)2 undergoes a double Sodium Chloride And Lead Ii Nitrate Formula The products of the reaction are an aqueous solution of sodium nitrate and a. The balanced equation will be calculated along with the. In this class practical, students react two soluble metal salts to produce one soluble salt and one insoluble salt. When aqueous lead nitrate (pb (no 3) 2) and aqueous or solid sodium chloride (hcl) are reacted, lead. Sodium Chloride And Lead Ii Nitrate Formula.
From www.slideserve.com
PPT Solubility Rules PowerPoint Presentation, free download ID4934641 Sodium Chloride And Lead Ii Nitrate Formula Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate. Enter an equation of an ionic chemical equation and press the balance button. In this class practical, students react two soluble metal salts to produce one soluble salt and one insoluble salt. The products of the reaction are an aqueous solution. Sodium Chloride And Lead Ii Nitrate Formula.
From www.chegg.com
Solved When copper (II) chloride reacts with sodium nitrate, Sodium Chloride And Lead Ii Nitrate Formula When sodium chloride is added to the lead nitrate solution a precipitate of lead chloride and sodium nitrate is formed. Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate. But for the net ionic equation, we represent only the net, macroscopic chemical change: In this class practical, students react two. Sodium Chloride And Lead Ii Nitrate Formula.
From exoncvmev.blob.core.windows.net
Lead Ii Nitrate And Sodium Chloride Balanced Equation at Janice Timmons Sodium Chloride And Lead Ii Nitrate Formula Enter an equation of an ionic chemical equation and press the balance button. Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate. In this class practical, students react two soluble metal salts to produce one soluble salt and one insoluble salt. The balanced equation will be calculated along with the.. Sodium Chloride And Lead Ii Nitrate Formula.
From www.numerade.com
SOLVED Solid nickel reacts with aqueous lead (II) nitrate to form Sodium Chloride And Lead Ii Nitrate Formula When sodium chloride is added to the lead nitrate solution a precipitate of lead chloride and sodium nitrate is formed. The products of the reaction are an aqueous solution of sodium nitrate and a. Enter an equation of an ionic chemical equation and press the balance button. When aqueous lead nitrate (pb (no 3) 2) and aqueous or solid sodium. Sodium Chloride And Lead Ii Nitrate Formula.
From exoncvmev.blob.core.windows.net
Lead Ii Nitrate And Sodium Chloride Balanced Equation at Janice Timmons Sodium Chloride And Lead Ii Nitrate Formula The products of the reaction are an aqueous solution of sodium nitrate and a. Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate. But for the net ionic equation, we represent only the net, macroscopic chemical change: When aqueous lead nitrate (pb (no 3) 2) and aqueous or solid sodium. Sodium Chloride And Lead Ii Nitrate Formula.