Standard Heat Of Formation . Learn the definition, equation and values of standard enthalpy of formation, the energy change when one mole of a substance is formed from. The standard state heat of formation for the elemental form of each atom is zero. The elemental form of each atom is that with the lowest enthalpy in the standard state. Learn how to use standard enthalpies of formation (δho f) to calculate enthalpies of reaction (δhrxn) for any chemical reaction. Learn what standard enthalpy of formation is, how to calculate it, and how to use it for chemical reactions. The standard enthalpy of formation, δh° f, is the enthalpy change accompanying the formation of 1 mole of a substance from the elements in their. See examples, characteristics, and a table of values for common substances. Learn how to calculate and use standard enthalpy of formation, an enthalpy change for a reaction that forms 1 mole of a substance from free. Heat of formation, the amount of heat absorbed or evolved when one mole of a compound is formed from its constituent elements, each substance. The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements. Find definitions, examples, key equations, and.
from www.chegg.com
Heat of formation, the amount of heat absorbed or evolved when one mole of a compound is formed from its constituent elements, each substance. Learn the definition, equation and values of standard enthalpy of formation, the energy change when one mole of a substance is formed from. Find definitions, examples, key equations, and. The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements. The standard state heat of formation for the elemental form of each atom is zero. The elemental form of each atom is that with the lowest enthalpy in the standard state. See examples, characteristics, and a table of values for common substances. The standard enthalpy of formation, δh° f, is the enthalpy change accompanying the formation of 1 mole of a substance from the elements in their. Learn how to calculate and use standard enthalpy of formation, an enthalpy change for a reaction that forms 1 mole of a substance from free. Learn how to use standard enthalpies of formation (δho f) to calculate enthalpies of reaction (δhrxn) for any chemical reaction.
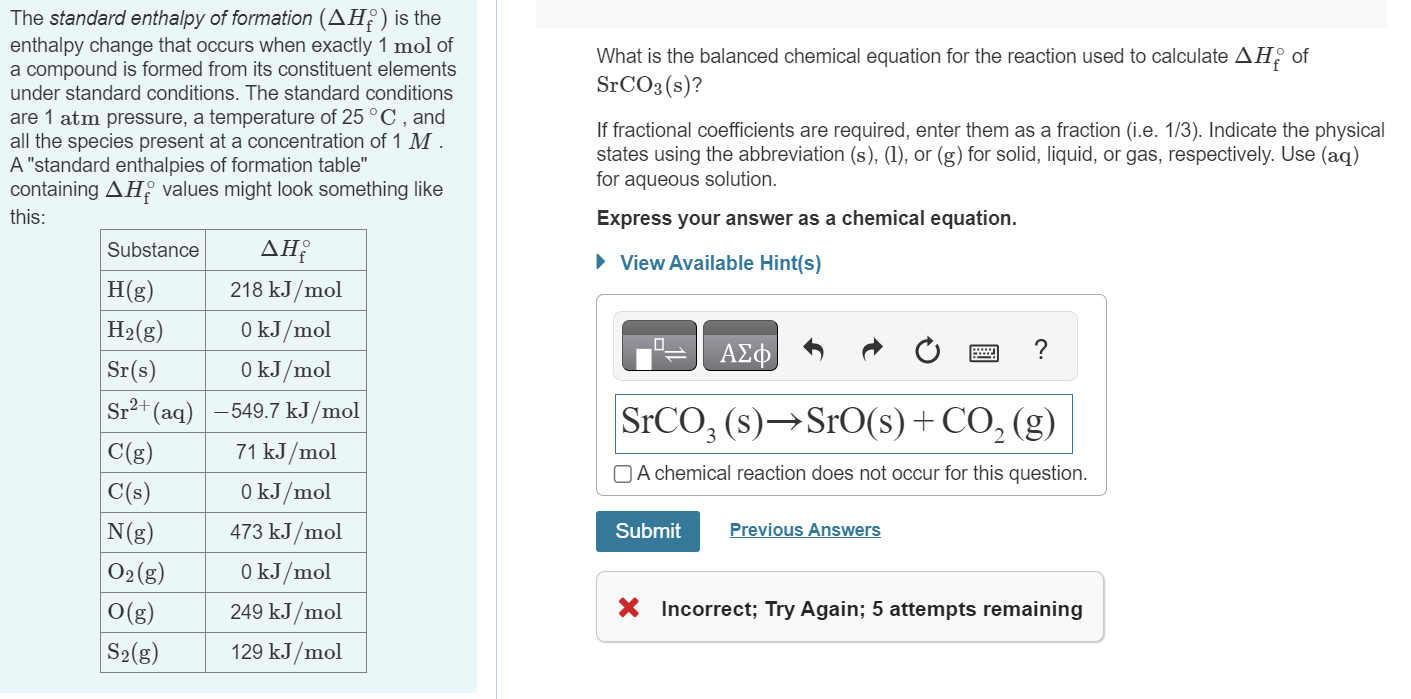
Solved The standard enthalpy of formation (ΔHf∘) is the
Standard Heat Of Formation Heat of formation, the amount of heat absorbed or evolved when one mole of a compound is formed from its constituent elements, each substance. Heat of formation, the amount of heat absorbed or evolved when one mole of a compound is formed from its constituent elements, each substance. Learn how to calculate and use standard enthalpy of formation, an enthalpy change for a reaction that forms 1 mole of a substance from free. The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements. Learn the definition, equation and values of standard enthalpy of formation, the energy change when one mole of a substance is formed from. Learn what standard enthalpy of formation is, how to calculate it, and how to use it for chemical reactions. Learn how to use standard enthalpies of formation (δho f) to calculate enthalpies of reaction (δhrxn) for any chemical reaction. The elemental form of each atom is that with the lowest enthalpy in the standard state. Find definitions, examples, key equations, and. The standard state heat of formation for the elemental form of each atom is zero. See examples, characteristics, and a table of values for common substances. The standard enthalpy of formation, δh° f, is the enthalpy change accompanying the formation of 1 mole of a substance from the elements in their.
From haipernews.com
How To Calculate Heat In A Reaction Haiper Standard Heat Of Formation Learn what standard enthalpy of formation is, how to calculate it, and how to use it for chemical reactions. Learn how to use standard enthalpies of formation (δho f) to calculate enthalpies of reaction (δhrxn) for any chemical reaction. The elemental form of each atom is that with the lowest enthalpy in the standard state. Learn how to calculate and. Standard Heat Of Formation.
From mavink.com
Enthalpy Of Formation Symbol Standard Heat Of Formation The standard state heat of formation for the elemental form of each atom is zero. The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements. Learn how to calculate and use standard enthalpy of formation, an enthalpy change for a reaction that forms 1 mole of a substance from free. Heat. Standard Heat Of Formation.
From miracleabbcortez.blogspot.com
Heat of Reaction Formula MiracleabbCortez Standard Heat Of Formation Learn how to calculate and use standard enthalpy of formation, an enthalpy change for a reaction that forms 1 mole of a substance from free. Find definitions, examples, key equations, and. Learn how to use standard enthalpies of formation (δho f) to calculate enthalpies of reaction (δhrxn) for any chemical reaction. The enthalpy of formation (\(δh_{f}\)) is the enthalpy change. Standard Heat Of Formation.
From www.chegg.com
Solved The standard enthalpy of formation (ΔHf∘) is the Standard Heat Of Formation The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements. Learn what standard enthalpy of formation is, how to calculate it, and how to use it for chemical reactions. The standard state heat of formation for the elemental form of each atom is zero. Find definitions, examples, key equations, and. Heat. Standard Heat Of Formation.
From www.chegg.com
Solved Table 2.2 Heat of formation Дh in kJ/mole (at 25°C Standard Heat Of Formation Learn the definition, equation and values of standard enthalpy of formation, the energy change when one mole of a substance is formed from. See examples, characteristics, and a table of values for common substances. Learn how to use standard enthalpies of formation (δho f) to calculate enthalpies of reaction (δhrxn) for any chemical reaction. The standard enthalpy of formation, δh°. Standard Heat Of Formation.
From mungfali.com
Enthalpies Of Formation Chart Standard Heat Of Formation Heat of formation, the amount of heat absorbed or evolved when one mole of a compound is formed from its constituent elements, each substance. Learn how to use standard enthalpies of formation (δho f) to calculate enthalpies of reaction (δhrxn) for any chemical reaction. The standard state heat of formation for the elemental form of each atom is zero. Learn. Standard Heat Of Formation.
From slideplayer.com
Chapter 17 “Thermochemistry” ppt download Standard Heat Of Formation The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements. See examples, characteristics, and a table of values for common substances. Learn how to use standard enthalpies of formation (δho f) to calculate enthalpies of reaction (δhrxn) for any chemical reaction. The standard state heat of formation for the elemental form. Standard Heat Of Formation.
From mavink.com
Standard Enthalpy Chart Standard Heat Of Formation Find definitions, examples, key equations, and. Learn how to calculate and use standard enthalpy of formation, an enthalpy change for a reaction that forms 1 mole of a substance from free. Learn how to use standard enthalpies of formation (δho f) to calculate enthalpies of reaction (δhrxn) for any chemical reaction. The enthalpy of formation (\(δh_{f}\)) is the enthalpy change. Standard Heat Of Formation.
From mavink.com
Enthalpy Of Formation Symbol Standard Heat Of Formation See examples, characteristics, and a table of values for common substances. The elemental form of each atom is that with the lowest enthalpy in the standard state. The standard state heat of formation for the elemental form of each atom is zero. Learn how to calculate and use standard enthalpy of formation, an enthalpy change for a reaction that forms. Standard Heat Of Formation.
From materiallistgaskell.z21.web.core.windows.net
Heat Of Formation Equations Standard Heat Of Formation Learn how to use standard enthalpies of formation (δho f) to calculate enthalpies of reaction (δhrxn) for any chemical reaction. The elemental form of each atom is that with the lowest enthalpy in the standard state. Learn how to calculate and use standard enthalpy of formation, an enthalpy change for a reaction that forms 1 mole of a substance from. Standard Heat Of Formation.
From slideplayer.com
Chapter 10 “Thermochemistry” ppt download Standard Heat Of Formation See examples, characteristics, and a table of values for common substances. The standard enthalpy of formation, δh° f, is the enthalpy change accompanying the formation of 1 mole of a substance from the elements in their. Learn what standard enthalpy of formation is, how to calculate it, and how to use it for chemical reactions. Heat of formation, the amount. Standard Heat Of Formation.
From schoolworkhelper.net
Standard Enthalpies of Formation SchoolWorkHelper Standard Heat Of Formation The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements. Learn how to use standard enthalpies of formation (δho f) to calculate enthalpies of reaction (δhrxn) for any chemical reaction. The standard state heat of formation for the elemental form of each atom is zero. See examples, characteristics, and a table. Standard Heat Of Formation.
From worksheetzonepatine.z14.web.core.windows.net
Heat Of Formation Chart Standard Heat Of Formation The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements. Learn how to calculate and use standard enthalpy of formation, an enthalpy change for a reaction that forms 1 mole of a substance from free. The standard state heat of formation for the elemental form of each atom is zero. Heat. Standard Heat Of Formation.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID314871 Standard Heat Of Formation See examples, characteristics, and a table of values for common substances. Heat of formation, the amount of heat absorbed or evolved when one mole of a compound is formed from its constituent elements, each substance. The elemental form of each atom is that with the lowest enthalpy in the standard state. Learn the definition, equation and values of standard enthalpy. Standard Heat Of Formation.
From mavink.com
Enthalpy Of Formation Equation Standard Heat Of Formation Heat of formation, the amount of heat absorbed or evolved when one mole of a compound is formed from its constituent elements, each substance. Find definitions, examples, key equations, and. Learn how to use standard enthalpies of formation (δho f) to calculate enthalpies of reaction (δhrxn) for any chemical reaction. Learn the definition, equation and values of standard enthalpy of. Standard Heat Of Formation.
From slideplayer.com
Heat in Changes of State and Calculating Heat of Reaction ppt download Standard Heat Of Formation The elemental form of each atom is that with the lowest enthalpy in the standard state. The standard state heat of formation for the elemental form of each atom is zero. Learn how to calculate and use standard enthalpy of formation, an enthalpy change for a reaction that forms 1 mole of a substance from free. Learn what standard enthalpy. Standard Heat Of Formation.
From www.slideserve.com
PPT STANDARD HEAT OF FORMATION ΔH 0 f or ΔH θ f PowerPoint Presentation ID3181539 Standard Heat Of Formation Find definitions, examples, key equations, and. The elemental form of each atom is that with the lowest enthalpy in the standard state. The standard enthalpy of formation, δh° f, is the enthalpy change accompanying the formation of 1 mole of a substance from the elements in their. Heat of formation, the amount of heat absorbed or evolved when one mole. Standard Heat Of Formation.
From www.slideserve.com
PPT STANDARD HEAT OF FORMATION ΔH 0 f or ΔH θ f PowerPoint Presentation ID3181539 Standard Heat Of Formation Heat of formation, the amount of heat absorbed or evolved when one mole of a compound is formed from its constituent elements, each substance. Find definitions, examples, key equations, and. Learn how to calculate and use standard enthalpy of formation, an enthalpy change for a reaction that forms 1 mole of a substance from free. The enthalpy of formation (\(δh_{f}\)). Standard Heat Of Formation.
From mungfali.com
Standard Enthalpy Of Formation Equation Standard Heat Of Formation The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements. Find definitions, examples, key equations, and. Heat of formation, the amount of heat absorbed or evolved when one mole of a compound is formed from its constituent elements, each substance. The elemental form of each atom is that with the lowest. Standard Heat Of Formation.
From www.studocu.com
Standard Enthalpy of Formation Table Standard Enthalpy of Formation* for Various Compounds Standard Heat Of Formation Heat of formation, the amount of heat absorbed or evolved when one mole of a compound is formed from its constituent elements, each substance. The standard enthalpy of formation, δh° f, is the enthalpy change accompanying the formation of 1 mole of a substance from the elements in their. Learn how to calculate and use standard enthalpy of formation, an. Standard Heat Of Formation.
From rayb78.github.io
Heat Of Formation Chart Standard Heat Of Formation See examples, characteristics, and a table of values for common substances. The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements. The elemental form of each atom is that with the lowest enthalpy in the standard state. Heat of formation, the amount of heat absorbed or evolved when one mole of. Standard Heat Of Formation.
From quizzlistreplevies.z13.web.core.windows.net
Heat Of Formation Equations Standard Heat Of Formation Find definitions, examples, key equations, and. Heat of formation, the amount of heat absorbed or evolved when one mole of a compound is formed from its constituent elements, each substance. The standard enthalpy of formation, δh° f, is the enthalpy change accompanying the formation of 1 mole of a substance from the elements in their. Learn the definition, equation and. Standard Heat Of Formation.
From mavink.com
Standard Enthalpy Chart Standard Heat Of Formation The standard enthalpy of formation, δh° f, is the enthalpy change accompanying the formation of 1 mole of a substance from the elements in their. Learn what standard enthalpy of formation is, how to calculate it, and how to use it for chemical reactions. Learn how to use standard enthalpies of formation (δho f) to calculate enthalpies of reaction (δhrxn). Standard Heat Of Formation.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free download ID4097208 Standard Heat Of Formation Learn the definition, equation and values of standard enthalpy of formation, the energy change when one mole of a substance is formed from. The standard enthalpy of formation, δh° f, is the enthalpy change accompanying the formation of 1 mole of a substance from the elements in their. The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the. Standard Heat Of Formation.
From www.slideserve.com
PPT Chemistry 17.4 PowerPoint Presentation, free download ID2772524 Standard Heat Of Formation The standard enthalpy of formation, δh° f, is the enthalpy change accompanying the formation of 1 mole of a substance from the elements in their. The elemental form of each atom is that with the lowest enthalpy in the standard state. Learn the definition, equation and values of standard enthalpy of formation, the energy change when one mole of a. Standard Heat Of Formation.
From stahonorschemistry.weebly.com
III Calculating Enthalpies STA Form IV Honors Chemistry Thermochemistry Unit Standard Heat Of Formation The standard enthalpy of formation, δh° f, is the enthalpy change accompanying the formation of 1 mole of a substance from the elements in their. Learn how to use standard enthalpies of formation (δho f) to calculate enthalpies of reaction (δhrxn) for any chemical reaction. Learn how to calculate and use standard enthalpy of formation, an enthalpy change for a. Standard Heat Of Formation.
From www.slideserve.com
PPT Energy Transformations PowerPoint Presentation, free download ID7001222 Standard Heat Of Formation The elemental form of each atom is that with the lowest enthalpy in the standard state. Learn what standard enthalpy of formation is, how to calculate it, and how to use it for chemical reactions. Learn how to use standard enthalpies of formation (δho f) to calculate enthalpies of reaction (δhrxn) for any chemical reaction. The standard enthalpy of formation,. Standard Heat Of Formation.
From www.chegg.com
Solved Use the standard enthalpies of formation in the table Standard Heat Of Formation Learn how to use standard enthalpies of formation (δho f) to calculate enthalpies of reaction (δhrxn) for any chemical reaction. Learn what standard enthalpy of formation is, how to calculate it, and how to use it for chemical reactions. Learn the definition, equation and values of standard enthalpy of formation, the energy change when one mole of a substance is. Standard Heat Of Formation.
From duanerafanan.blogspot.com
DUANE HESS'S LAW Standard Heat Of Formation The elemental form of each atom is that with the lowest enthalpy in the standard state. Find definitions, examples, key equations, and. Learn what standard enthalpy of formation is, how to calculate it, and how to use it for chemical reactions. Learn how to use standard enthalpies of formation (δho f) to calculate enthalpies of reaction (δhrxn) for any chemical. Standard Heat Of Formation.
From www.nagwa.com
Question Video Calculating the Standard Enthalpy of Formation for Heptane Using Standard Standard Heat Of Formation Learn how to calculate and use standard enthalpy of formation, an enthalpy change for a reaction that forms 1 mole of a substance from free. The elemental form of each atom is that with the lowest enthalpy in the standard state. Learn what standard enthalpy of formation is, how to calculate it, and how to use it for chemical reactions.. Standard Heat Of Formation.
From storage.googleapis.com
Standard Enthalpy Of Formation Sulfuric Acid Standard Heat Of Formation Learn how to calculate and use standard enthalpy of formation, an enthalpy change for a reaction that forms 1 mole of a substance from free. The standard state heat of formation for the elemental form of each atom is zero. Find definitions, examples, key equations, and. The elemental form of each atom is that with the lowest enthalpy in the. Standard Heat Of Formation.
From www.youtube.com
Standard heat of formation problem / Heat of formation formation enthalpy YouTube Standard Heat Of Formation The standard enthalpy of formation, δh° f, is the enthalpy change accompanying the formation of 1 mole of a substance from the elements in their. Heat of formation, the amount of heat absorbed or evolved when one mole of a compound is formed from its constituent elements, each substance. The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies. Standard Heat Of Formation.
From byjus.com
43. Calculate standard heat of formation of CS2. Given that standard heat of combustion of C, S Standard Heat Of Formation Learn how to calculate and use standard enthalpy of formation, an enthalpy change for a reaction that forms 1 mole of a substance from free. See examples, characteristics, and a table of values for common substances. The standard state heat of formation for the elemental form of each atom is zero. The enthalpy of formation (\(δh_{f}\)) is the enthalpy change. Standard Heat Of Formation.
From printableliblayette.z13.web.core.windows.net
Heat Of Formation Worksheet Standard Heat Of Formation The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements. The standard enthalpy of formation, δh° f, is the enthalpy change accompanying the formation of 1 mole of a substance from the elements in their. The elemental form of each atom is that with the lowest enthalpy in the standard state.. Standard Heat Of Formation.
From brunofuga.adv.br
Standard Enthalpy Of Formation Definition, Table, Equation, 46 OFF Standard Heat Of Formation Learn how to calculate and use standard enthalpy of formation, an enthalpy change for a reaction that forms 1 mole of a substance from free. Learn the definition, equation and values of standard enthalpy of formation, the energy change when one mole of a substance is formed from. Heat of formation, the amount of heat absorbed or evolved when one. Standard Heat Of Formation.