How Does Water Vapor Pressure Work . the pressure exerted by a vapor in dynamic equilibrium with a liquid is the equilibrium vapor pressure of the liquid. Strong intermolecular forces produce a lower rate of. vapor pressure is a measure of the pressure exerted by a gas above a liquid in a sealed container. the vapor pressure of water is the pressure at which water vapor is in thermodynamic equilibrium with its condensed state. If a liquid is in an open container, however, most of the molecules that escape into the vapor phase will not collide with the surface of the liquid and return to the liquid phase. the vapor pressure of water is the pressure at which the gas phase is in equilibrium with the liquid phase. the vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); That is, the pressure of the vapor resulting from.
from sciencepolicy.colorado.edu
vapor pressure is a measure of the pressure exerted by a gas above a liquid in a sealed container. the vapor pressure of water is the pressure at which the gas phase is in equilibrium with the liquid phase. the vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); the pressure exerted by a vapor in dynamic equilibrium with a liquid is the equilibrium vapor pressure of the liquid. That is, the pressure of the vapor resulting from. Strong intermolecular forces produce a lower rate of. If a liquid is in an open container, however, most of the molecules that escape into the vapor phase will not collide with the surface of the liquid and return to the liquid phase. the vapor pressure of water is the pressure at which water vapor is in thermodynamic equilibrium with its condensed state.
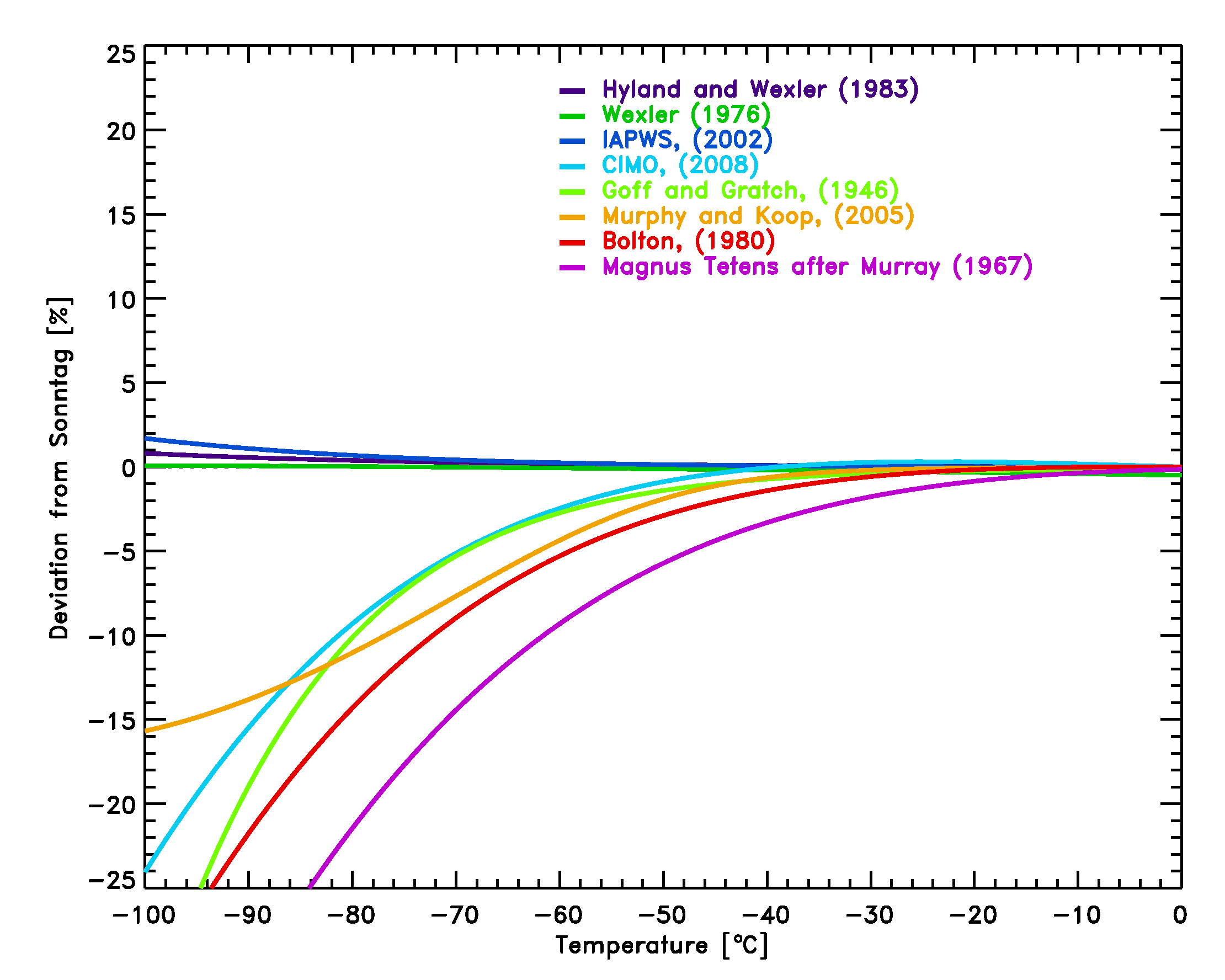
Water Vapor Pressure Formulations
How Does Water Vapor Pressure Work vapor pressure is a measure of the pressure exerted by a gas above a liquid in a sealed container. If a liquid is in an open container, however, most of the molecules that escape into the vapor phase will not collide with the surface of the liquid and return to the liquid phase. the pressure exerted by a vapor in dynamic equilibrium with a liquid is the equilibrium vapor pressure of the liquid. Strong intermolecular forces produce a lower rate of. the vapor pressure of water is the pressure at which water vapor is in thermodynamic equilibrium with its condensed state. the vapor pressure of water is the pressure at which the gas phase is in equilibrium with the liquid phase. That is, the pressure of the vapor resulting from. vapor pressure is a measure of the pressure exerted by a gas above a liquid in a sealed container. the vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid);
From www.youtube.com
The Pressure is On! A Beginner's Guide to Water Vapor Pressure YouTube How Does Water Vapor Pressure Work vapor pressure is a measure of the pressure exerted by a gas above a liquid in a sealed container. the pressure exerted by a vapor in dynamic equilibrium with a liquid is the equilibrium vapor pressure of the liquid. the vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid);. How Does Water Vapor Pressure Work.
From fyolwfokx.blob.core.windows.net
Water Well Pressure Tank Fittings at John Waller blog How Does Water Vapor Pressure Work the vapor pressure of water is the pressure at which water vapor is in thermodynamic equilibrium with its condensed state. the vapor pressure of water is the pressure at which the gas phase is in equilibrium with the liquid phase. the vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or. How Does Water Vapor Pressure Work.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID5080574 How Does Water Vapor Pressure Work the pressure exerted by a vapor in dynamic equilibrium with a liquid is the equilibrium vapor pressure of the liquid. If a liquid is in an open container, however, most of the molecules that escape into the vapor phase will not collide with the surface of the liquid and return to the liquid phase. That is, the pressure of. How Does Water Vapor Pressure Work.
From www.slideserve.com
PPT 6 Gases PowerPoint Presentation, free download ID352441 How Does Water Vapor Pressure Work If a liquid is in an open container, however, most of the molecules that escape into the vapor phase will not collide with the surface of the liquid and return to the liquid phase. the vapor pressure of water is the pressure at which water vapor is in thermodynamic equilibrium with its condensed state. the vapor pressure of. How Does Water Vapor Pressure Work.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID7038735 How Does Water Vapor Pressure Work the vapor pressure of water is the pressure at which the gas phase is in equilibrium with the liquid phase. That is, the pressure of the vapor resulting from. vapor pressure is a measure of the pressure exerted by a gas above a liquid in a sealed container. If a liquid is in an open container, however, most. How Does Water Vapor Pressure Work.
From www.wikihow.com
3 Easy Ways to Calculate Vapor Pressure (with Pictures) How Does Water Vapor Pressure Work That is, the pressure of the vapor resulting from. the vapor pressure of water is the pressure at which the gas phase is in equilibrium with the liquid phase. If a liquid is in an open container, however, most of the molecules that escape into the vapor phase will not collide with the surface of the liquid and return. How Does Water Vapor Pressure Work.
From sciencepolicy.colorado.edu
Water Vapor Pressure Formulations How Does Water Vapor Pressure Work the vapor pressure of water is the pressure at which water vapor is in thermodynamic equilibrium with its condensed state. vapor pressure is a measure of the pressure exerted by a gas above a liquid in a sealed container. the vapor pressure of water is the pressure at which the gas phase is in equilibrium with the. How Does Water Vapor Pressure Work.
From ecoursesonline.iasri.res.in
FM LESSON 3. PROPERTIES OF FLUID How Does Water Vapor Pressure Work That is, the pressure of the vapor resulting from. the vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); If a liquid is in an open container, however, most of the molecules that escape into the vapor phase will not collide with the surface of the liquid and return to the. How Does Water Vapor Pressure Work.
From sciencenotes.org
Vapor Pressure Definition and How to Calculate It How Does Water Vapor Pressure Work That is, the pressure of the vapor resulting from. the vapor pressure of water is the pressure at which water vapor is in thermodynamic equilibrium with its condensed state. If a liquid is in an open container, however, most of the molecules that escape into the vapor phase will not collide with the surface of the liquid and return. How Does Water Vapor Pressure Work.
From www.slideserve.com
PPT Gas Exchange in Lungs PowerPoint Presentation, free download ID How Does Water Vapor Pressure Work the vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); the pressure exerted by a vapor in dynamic equilibrium with a liquid is the equilibrium vapor pressure of the liquid. Strong intermolecular forces produce a lower rate of. That is, the pressure of the vapor resulting from. vapor pressure. How Does Water Vapor Pressure Work.
From www.youtube.com
Evaporation, Vapor Pressure and Boiling YouTube How Does Water Vapor Pressure Work That is, the pressure of the vapor resulting from. the vapor pressure of water is the pressure at which the gas phase is in equilibrium with the liquid phase. Strong intermolecular forces produce a lower rate of. the vapor pressure of water is the pressure at which water vapor is in thermodynamic equilibrium with its condensed state. . How Does Water Vapor Pressure Work.
From www.wikiwand.com
Vapor pressure Wikiwand How Does Water Vapor Pressure Work the vapor pressure of water is the pressure at which water vapor is in thermodynamic equilibrium with its condensed state. Strong intermolecular forces produce a lower rate of. the vapor pressure of water is the pressure at which the gas phase is in equilibrium with the liquid phase. vapor pressure is a measure of the pressure exerted. How Does Water Vapor Pressure Work.
From socratic.org
Explain how each of the following affects the vapour pressure of a How Does Water Vapor Pressure Work the vapor pressure of water is the pressure at which the gas phase is in equilibrium with the liquid phase. the pressure exerted by a vapor in dynamic equilibrium with a liquid is the equilibrium vapor pressure of the liquid. Strong intermolecular forces produce a lower rate of. If a liquid is in an open container, however, most. How Does Water Vapor Pressure Work.
From www.youtube.com
vapor pressure explained YouTube How Does Water Vapor Pressure Work If a liquid is in an open container, however, most of the molecules that escape into the vapor phase will not collide with the surface of the liquid and return to the liquid phase. the vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); the vapor pressure of water is. How Does Water Vapor Pressure Work.
From saylordotorg.github.io
Vapor Pressure How Does Water Vapor Pressure Work That is, the pressure of the vapor resulting from. Strong intermolecular forces produce a lower rate of. the vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); the vapor pressure of water is the pressure at which the gas phase is in equilibrium with the liquid phase. the pressure. How Does Water Vapor Pressure Work.
From www.youtube.com
Vapor Pressure, Equilibrium Vapor Pressure, and Relative Humidity YouTube How Does Water Vapor Pressure Work If a liquid is in an open container, however, most of the molecules that escape into the vapor phase will not collide with the surface of the liquid and return to the liquid phase. That is, the pressure of the vapor resulting from. the pressure exerted by a vapor in dynamic equilibrium with a liquid is the equilibrium vapor. How Does Water Vapor Pressure Work.
From study.com
Vapor Pressure Definition, Formula & Examples Lesson How Does Water Vapor Pressure Work the vapor pressure of water is the pressure at which the gas phase is in equilibrium with the liquid phase. the vapor pressure of water is the pressure at which water vapor is in thermodynamic equilibrium with its condensed state. the pressure exerted by a vapor in dynamic equilibrium with a liquid is the equilibrium vapor pressure. How Does Water Vapor Pressure Work.
From general.chemistrysteps.com
Vapor Pressure Lowering Chemistry Steps How Does Water Vapor Pressure Work vapor pressure is a measure of the pressure exerted by a gas above a liquid in a sealed container. the vapor pressure of water is the pressure at which water vapor is in thermodynamic equilibrium with its condensed state. the vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid);. How Does Water Vapor Pressure Work.
From www.slideserve.com
PPT Solution properties PowerPoint Presentation, free download ID How Does Water Vapor Pressure Work the pressure exerted by a vapor in dynamic equilibrium with a liquid is the equilibrium vapor pressure of the liquid. the vapor pressure of water is the pressure at which water vapor is in thermodynamic equilibrium with its condensed state. vapor pressure is a measure of the pressure exerted by a gas above a liquid in a. How Does Water Vapor Pressure Work.
From general.chemistrysteps.com
Vapor Pressure Lowering Chemistry Steps How Does Water Vapor Pressure Work the vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); the pressure exerted by a vapor in dynamic equilibrium with a liquid is the equilibrium vapor pressure of the liquid. the vapor pressure of water is the pressure at which the gas phase is in equilibrium with the liquid. How Does Water Vapor Pressure Work.
From general.chemistrysteps.com
Vapor Pressure Lowering Chemistry Steps How Does Water Vapor Pressure Work If a liquid is in an open container, however, most of the molecules that escape into the vapor phase will not collide with the surface of the liquid and return to the liquid phase. vapor pressure is a measure of the pressure exerted by a gas above a liquid in a sealed container. the vapor pressure of water. How Does Water Vapor Pressure Work.
From www.youtube.com
Vapor Pressure and Boiling YouTube How Does Water Vapor Pressure Work That is, the pressure of the vapor resulting from. the pressure exerted by a vapor in dynamic equilibrium with a liquid is the equilibrium vapor pressure of the liquid. If a liquid is in an open container, however, most of the molecules that escape into the vapor phase will not collide with the surface of the liquid and return. How Does Water Vapor Pressure Work.
From saylordotorg.github.io
Vapor Pressure How Does Water Vapor Pressure Work the vapor pressure of water is the pressure at which the gas phase is in equilibrium with the liquid phase. the vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); the vapor pressure of water is the pressure at which water vapor is in thermodynamic equilibrium with its condensed. How Does Water Vapor Pressure Work.
From www.slideserve.com
PPT Why . . . PowerPoint Presentation, free download ID2250075 How Does Water Vapor Pressure Work Strong intermolecular forces produce a lower rate of. That is, the pressure of the vapor resulting from. the vapor pressure of water is the pressure at which water vapor is in thermodynamic equilibrium with its condensed state. the pressure exerted by a vapor in dynamic equilibrium with a liquid is the equilibrium vapor pressure of the liquid. . How Does Water Vapor Pressure Work.
From engineerexcel.com
Vapor Pressure of Water Explained EngineerExcel How Does Water Vapor Pressure Work the vapor pressure of water is the pressure at which water vapor is in thermodynamic equilibrium with its condensed state. vapor pressure is a measure of the pressure exerted by a gas above a liquid in a sealed container. the vapor pressure of water is the pressure at which the gas phase is in equilibrium with the. How Does Water Vapor Pressure Work.
From www.chegg.com
Solved Shown below is the vapor pressure curve for water How Does Water Vapor Pressure Work That is, the pressure of the vapor resulting from. Strong intermolecular forces produce a lower rate of. the vapor pressure of water is the pressure at which the gas phase is in equilibrium with the liquid phase. the pressure exerted by a vapor in dynamic equilibrium with a liquid is the equilibrium vapor pressure of the liquid. . How Does Water Vapor Pressure Work.
From pediaa.com
Difference Between Vapor Pressure and Boiling Point Definition How Does Water Vapor Pressure Work That is, the pressure of the vapor resulting from. If a liquid is in an open container, however, most of the molecules that escape into the vapor phase will not collide with the surface of the liquid and return to the liquid phase. the vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid. How Does Water Vapor Pressure Work.
From www.researchgate.net
1 Vapor pressure of water Download Scientific Diagram How Does Water Vapor Pressure Work If a liquid is in an open container, however, most of the molecules that escape into the vapor phase will not collide with the surface of the liquid and return to the liquid phase. the vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Strong intermolecular forces produce a lower rate. How Does Water Vapor Pressure Work.
From engineerexcel.com
Vapor Pressure of Water Explained EngineerExcel How Does Water Vapor Pressure Work That is, the pressure of the vapor resulting from. the pressure exerted by a vapor in dynamic equilibrium with a liquid is the equilibrium vapor pressure of the liquid. If a liquid is in an open container, however, most of the molecules that escape into the vapor phase will not collide with the surface of the liquid and return. How Does Water Vapor Pressure Work.
From www.slideserve.com
PPT VAPOR PRESSURE OF WATER PowerPoint Presentation, free download How Does Water Vapor Pressure Work If a liquid is in an open container, however, most of the molecules that escape into the vapor phase will not collide with the surface of the liquid and return to the liquid phase. the vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); the vapor pressure of water is. How Does Water Vapor Pressure Work.
From www.researchgate.net
Vapor pressure and density of water vapor as function of temperature How Does Water Vapor Pressure Work That is, the pressure of the vapor resulting from. Strong intermolecular forces produce a lower rate of. the vapor pressure of water is the pressure at which water vapor is in thermodynamic equilibrium with its condensed state. the pressure exerted by a vapor in dynamic equilibrium with a liquid is the equilibrium vapor pressure of the liquid. . How Does Water Vapor Pressure Work.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID2755893 How Does Water Vapor Pressure Work If a liquid is in an open container, however, most of the molecules that escape into the vapor phase will not collide with the surface of the liquid and return to the liquid phase. Strong intermolecular forces produce a lower rate of. the pressure exerted by a vapor in dynamic equilibrium with a liquid is the equilibrium vapor pressure. How Does Water Vapor Pressure Work.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID1025977 How Does Water Vapor Pressure Work the pressure exerted by a vapor in dynamic equilibrium with a liquid is the equilibrium vapor pressure of the liquid. the vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); If a liquid is in an open container, however, most of the molecules that escape into the vapor phase will. How Does Water Vapor Pressure Work.
From www.iqsdirectory.com
Ultrasonic Cleaning What Is It? How Does It Work? Types Of How Does Water Vapor Pressure Work the vapor pressure of water is the pressure at which water vapor is in thermodynamic equilibrium with its condensed state. Strong intermolecular forces produce a lower rate of. the vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); That is, the pressure of the vapor resulting from. vapor pressure. How Does Water Vapor Pressure Work.
From www.processsensing.com
Humidity Academy Theory 3 How Does Water Vapor Pressure Work the vapor pressure of water is the pressure at which the gas phase is in equilibrium with the liquid phase. Strong intermolecular forces produce a lower rate of. the vapor pressure of water is the pressure at which water vapor is in thermodynamic equilibrium with its condensed state. If a liquid is in an open container, however, most. How Does Water Vapor Pressure Work.