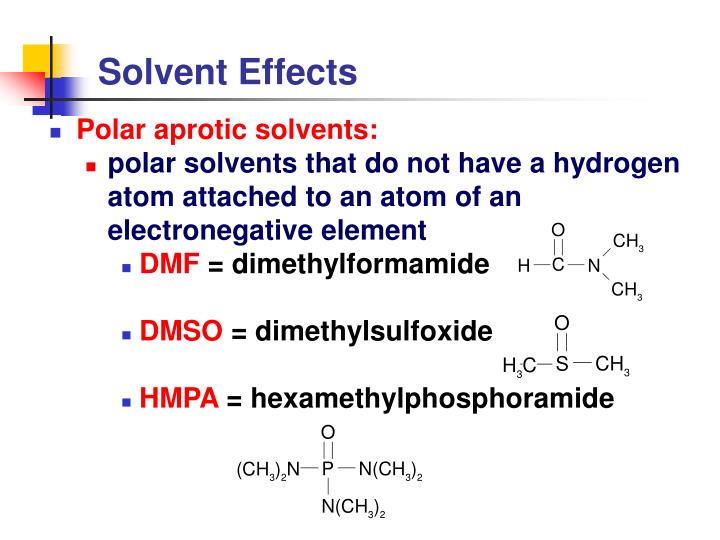
Example Of Solvent Effect . Solvent effects (how different solvents behave) and solvation (how solvents organize around a solute molecule) are very important to consider in thinking about acidity. The protic hydrogen can strongly interact with anions, whereas. According to this effect, the basicity of the solvents. Solvent effect refers to the impact of the solvent on the properties and behavior of organic molecules, including their electronic structure. The leveling effect is the ability of a solvent to enhance the strength of weak acids or bases to that of strong ones. Polar protic solvents are capable of making hydrogen bonding i.e. They contain a hydrogen connected to an electronegative atom and thus can make intermolecular. The impact of a solvent on the characteristics of acids and bases is known as the solvent leveling effect or solvent effect. Polar, protic solvents can stabilize ions through very strong intermolecular attractions.
from www.slideserve.com
Polar protic solvents are capable of making hydrogen bonding i.e. The leveling effect is the ability of a solvent to enhance the strength of weak acids or bases to that of strong ones. The protic hydrogen can strongly interact with anions, whereas. Polar, protic solvents can stabilize ions through very strong intermolecular attractions. According to this effect, the basicity of the solvents. Solvent effect refers to the impact of the solvent on the properties and behavior of organic molecules, including their electronic structure. They contain a hydrogen connected to an electronegative atom and thus can make intermolecular. The impact of a solvent on the characteristics of acids and bases is known as the solvent leveling effect or solvent effect. Solvent effects (how different solvents behave) and solvation (how solvents organize around a solute molecule) are very important to consider in thinking about acidity.
PPT Leaving Groups PowerPoint Presentation ID3540086
Example Of Solvent Effect The leveling effect is the ability of a solvent to enhance the strength of weak acids or bases to that of strong ones. According to this effect, the basicity of the solvents. Polar, protic solvents can stabilize ions through very strong intermolecular attractions. Polar protic solvents are capable of making hydrogen bonding i.e. Solvent effects (how different solvents behave) and solvation (how solvents organize around a solute molecule) are very important to consider in thinking about acidity. Solvent effect refers to the impact of the solvent on the properties and behavior of organic molecules, including their electronic structure. The leveling effect is the ability of a solvent to enhance the strength of weak acids or bases to that of strong ones. They contain a hydrogen connected to an electronegative atom and thus can make intermolecular. The impact of a solvent on the characteristics of acids and bases is known as the solvent leveling effect or solvent effect. The protic hydrogen can strongly interact with anions, whereas.
From www.slideserve.com
PPT Solvation PowerPoint Presentation, free download ID5189992 Example Of Solvent Effect The impact of a solvent on the characteristics of acids and bases is known as the solvent leveling effect or solvent effect. Solvent effects (how different solvents behave) and solvation (how solvents organize around a solute molecule) are very important to consider in thinking about acidity. They contain a hydrogen connected to an electronegative atom and thus can make intermolecular.. Example Of Solvent Effect.
From www.youtube.com
Effect of solvent in Nucleophilic Substitution Reaction YouTube Example Of Solvent Effect The leveling effect is the ability of a solvent to enhance the strength of weak acids or bases to that of strong ones. The impact of a solvent on the characteristics of acids and bases is known as the solvent leveling effect or solvent effect. Solvent effect refers to the impact of the solvent on the properties and behavior of. Example Of Solvent Effect.
From www.dreamstime.com
Solubility Vector Illustration. Labeled Solute, Solvent and Solution Example Of Solvent Effect The leveling effect is the ability of a solvent to enhance the strength of weak acids or bases to that of strong ones. According to this effect, the basicity of the solvents. Polar, protic solvents can stabilize ions through very strong intermolecular attractions. Polar protic solvents are capable of making hydrogen bonding i.e. The protic hydrogen can strongly interact with. Example Of Solvent Effect.
From www.slideserve.com
PPT Chapter 6 Alkyl Halides Nucleophilic Substitution and Example Of Solvent Effect The leveling effect is the ability of a solvent to enhance the strength of weak acids or bases to that of strong ones. Solvent effect refers to the impact of the solvent on the properties and behavior of organic molecules, including their electronic structure. Polar, protic solvents can stabilize ions through very strong intermolecular attractions. According to this effect, the. Example Of Solvent Effect.
From www.slideserve.com
PPT Organic Chemistry PowerPoint Presentation, free download ID375520 Example Of Solvent Effect Polar, protic solvents can stabilize ions through very strong intermolecular attractions. Solvent effects (how different solvents behave) and solvation (how solvents organize around a solute molecule) are very important to consider in thinking about acidity. They contain a hydrogen connected to an electronegative atom and thus can make intermolecular. The impact of a solvent on the characteristics of acids and. Example Of Solvent Effect.
From www.slideserve.com
PPT Reaction mechanisms PowerPoint Presentation, free download ID Example Of Solvent Effect According to this effect, the basicity of the solvents. The impact of a solvent on the characteristics of acids and bases is known as the solvent leveling effect or solvent effect. Solvent effect refers to the impact of the solvent on the properties and behavior of organic molecules, including their electronic structure. The leveling effect is the ability of a. Example Of Solvent Effect.
From www.youtube.com
Chapter 7 Solvent Effects YouTube Example Of Solvent Effect Polar protic solvents are capable of making hydrogen bonding i.e. Polar, protic solvents can stabilize ions through very strong intermolecular attractions. They contain a hydrogen connected to an electronegative atom and thus can make intermolecular. According to this effect, the basicity of the solvents. The impact of a solvent on the characteristics of acids and bases is known as the. Example Of Solvent Effect.
From www.chemistrysteps.com
The Role of Solvent in SN1, SN2, E1 and E2 Reactions Chemistry Steps Example Of Solvent Effect Solvent effect refers to the impact of the solvent on the properties and behavior of organic molecules, including their electronic structure. The leveling effect is the ability of a solvent to enhance the strength of weak acids or bases to that of strong ones. They contain a hydrogen connected to an electronegative atom and thus can make intermolecular. Polar, protic. Example Of Solvent Effect.
From www.slideserve.com
PPT Leaving Groups PowerPoint Presentation ID3540086 Example Of Solvent Effect The leveling effect is the ability of a solvent to enhance the strength of weak acids or bases to that of strong ones. The protic hydrogen can strongly interact with anions, whereas. According to this effect, the basicity of the solvents. They contain a hydrogen connected to an electronegative atom and thus can make intermolecular. The impact of a solvent. Example Of Solvent Effect.
From www.researchgate.net
Computational methods for modeling solvent effects on a solute. There Example Of Solvent Effect Polar protic solvents are capable of making hydrogen bonding i.e. Solvent effect refers to the impact of the solvent on the properties and behavior of organic molecules, including their electronic structure. They contain a hydrogen connected to an electronegative atom and thus can make intermolecular. The protic hydrogen can strongly interact with anions, whereas. According to this effect, the basicity. Example Of Solvent Effect.
From www.youtube.com
What is Effect of Solvent on UV Absorption Spectra Spectroscopy Example Of Solvent Effect Solvent effects (how different solvents behave) and solvation (how solvents organize around a solute molecule) are very important to consider in thinking about acidity. Solvent effect refers to the impact of the solvent on the properties and behavior of organic molecules, including their electronic structure. The leveling effect is the ability of a solvent to enhance the strength of weak. Example Of Solvent Effect.
From www.slideserve.com
PPT Solvents PowerPoint Presentation, free download ID420645 Example Of Solvent Effect Polar, protic solvents can stabilize ions through very strong intermolecular attractions. The leveling effect is the ability of a solvent to enhance the strength of weak acids or bases to that of strong ones. The impact of a solvent on the characteristics of acids and bases is known as the solvent leveling effect or solvent effect. According to this effect,. Example Of Solvent Effect.
From www.slideserve.com
PPT Solvent effects in RMG (Java + ) PowerPoint Presentation, free Example Of Solvent Effect They contain a hydrogen connected to an electronegative atom and thus can make intermolecular. Solvent effect refers to the impact of the solvent on the properties and behavior of organic molecules, including their electronic structure. Solvent effects (how different solvents behave) and solvation (how solvents organize around a solute molecule) are very important to consider in thinking about acidity. The. Example Of Solvent Effect.
From www.slideserve.com
PPT Reaction mechanisms PowerPoint Presentation, free download ID Example Of Solvent Effect Polar, protic solvents can stabilize ions through very strong intermolecular attractions. The impact of a solvent on the characteristics of acids and bases is known as the solvent leveling effect or solvent effect. Solvent effects (how different solvents behave) and solvation (how solvents organize around a solute molecule) are very important to consider in thinking about acidity. They contain a. Example Of Solvent Effect.
From www.wizeprep.com
Solvent Effects in Substitution Reactions Wize University Organic Example Of Solvent Effect Polar, protic solvents can stabilize ions through very strong intermolecular attractions. The protic hydrogen can strongly interact with anions, whereas. According to this effect, the basicity of the solvents. Solvent effects (how different solvents behave) and solvation (how solvents organize around a solute molecule) are very important to consider in thinking about acidity. The impact of a solvent on the. Example Of Solvent Effect.
From www.slideserve.com
PPT Solvent Effects PowerPoint Presentation, free download ID3080477 Example Of Solvent Effect They contain a hydrogen connected to an electronegative atom and thus can make intermolecular. Polar, protic solvents can stabilize ions through very strong intermolecular attractions. The protic hydrogen can strongly interact with anions, whereas. The impact of a solvent on the characteristics of acids and bases is known as the solvent leveling effect or solvent effect. Solvent effect refers to. Example Of Solvent Effect.
From slideplayer.com
Chemistry of Solutions ppt download Example Of Solvent Effect Solvent effects (how different solvents behave) and solvation (how solvents organize around a solute molecule) are very important to consider in thinking about acidity. The leveling effect is the ability of a solvent to enhance the strength of weak acids or bases to that of strong ones. The impact of a solvent on the characteristics of acids and bases is. Example Of Solvent Effect.
From www.youtube.com
Solvent Effects in UV Visible Spectroscopy YouTube Example Of Solvent Effect Polar protic solvents are capable of making hydrogen bonding i.e. Solvent effect refers to the impact of the solvent on the properties and behavior of organic molecules, including their electronic structure. They contain a hydrogen connected to an electronegative atom and thus can make intermolecular. According to this effect, the basicity of the solvents. The protic hydrogen can strongly interact. Example Of Solvent Effect.
From present5.com
Unit 6 How do we control chemical change Example Of Solvent Effect The leveling effect is the ability of a solvent to enhance the strength of weak acids or bases to that of strong ones. Polar protic solvents are capable of making hydrogen bonding i.e. Solvent effect refers to the impact of the solvent on the properties and behavior of organic molecules, including their electronic structure. They contain a hydrogen connected to. Example Of Solvent Effect.
From www.chemistrysteps.com
The Role of Solvent in SN1, SN2, E1 and E2 Reactions Chemistry Steps Example Of Solvent Effect Solvent effect refers to the impact of the solvent on the properties and behavior of organic molecules, including their electronic structure. Solvent effects (how different solvents behave) and solvation (how solvents organize around a solute molecule) are very important to consider in thinking about acidity. The protic hydrogen can strongly interact with anions, whereas. The impact of a solvent on. Example Of Solvent Effect.
From www.slideserve.com
PPT Emission spectroscopy (mainly fluorescence spectroscopy Example Of Solvent Effect Solvent effects (how different solvents behave) and solvation (how solvents organize around a solute molecule) are very important to consider in thinking about acidity. The protic hydrogen can strongly interact with anions, whereas. Solvent effect refers to the impact of the solvent on the properties and behavior of organic molecules, including their electronic structure. The leveling effect is the ability. Example Of Solvent Effect.
From www.slideserve.com
PPT Alkyl Halides PowerPoint Presentation, free download ID153595 Example Of Solvent Effect Solvent effects (how different solvents behave) and solvation (how solvents organize around a solute molecule) are very important to consider in thinking about acidity. According to this effect, the basicity of the solvents. The leveling effect is the ability of a solvent to enhance the strength of weak acids or bases to that of strong ones. The impact of a. Example Of Solvent Effect.
From www.slideserve.com
PPT Topic 12 Solutions PowerPoint Presentation, free download ID Example Of Solvent Effect They contain a hydrogen connected to an electronegative atom and thus can make intermolecular. Polar protic solvents are capable of making hydrogen bonding i.e. Solvent effect refers to the impact of the solvent on the properties and behavior of organic molecules, including their electronic structure. The leveling effect is the ability of a solvent to enhance the strength of weak. Example Of Solvent Effect.
From www.chemistrysteps.com
The Role of Solvent in SN1, SN2, E1 and E2 Reactions Chemistry Steps Example Of Solvent Effect Solvent effect refers to the impact of the solvent on the properties and behavior of organic molecules, including their electronic structure. The leveling effect is the ability of a solvent to enhance the strength of weak acids or bases to that of strong ones. Polar, protic solvents can stabilize ions through very strong intermolecular attractions. They contain a hydrogen connected. Example Of Solvent Effect.
From www.slideshare.net
Solvent Effects on Chemical Reaction PPT Example Of Solvent Effect The protic hydrogen can strongly interact with anions, whereas. Polar protic solvents are capable of making hydrogen bonding i.e. According to this effect, the basicity of the solvents. Solvent effects (how different solvents behave) and solvation (how solvents organize around a solute molecule) are very important to consider in thinking about acidity. The leveling effect is the ability of a. Example Of Solvent Effect.
From www.slideserve.com
PPT Solvent Effects PowerPoint Presentation, free download ID3080477 Example Of Solvent Effect Solvent effect refers to the impact of the solvent on the properties and behavior of organic molecules, including their electronic structure. According to this effect, the basicity of the solvents. The leveling effect is the ability of a solvent to enhance the strength of weak acids or bases to that of strong ones. Polar, protic solvents can stabilize ions through. Example Of Solvent Effect.
From chemistnotes.com
Solvent leveling effectDefinition, example Chemistry Notes Example Of Solvent Effect Polar, protic solvents can stabilize ions through very strong intermolecular attractions. The impact of a solvent on the characteristics of acids and bases is known as the solvent leveling effect or solvent effect. Solvent effects (how different solvents behave) and solvation (how solvents organize around a solute molecule) are very important to consider in thinking about acidity. Solvent effect refers. Example Of Solvent Effect.
From lornav-shot.blogspot.com
Solvents For Sn2 The Role Of Solvent In Sn1 Sn2 E1 And E2 Reactions Example Of Solvent Effect Solvent effects (how different solvents behave) and solvation (how solvents organize around a solute molecule) are very important to consider in thinking about acidity. The protic hydrogen can strongly interact with anions, whereas. Polar protic solvents are capable of making hydrogen bonding i.e. The leveling effect is the ability of a solvent to enhance the strength of weak acids or. Example Of Solvent Effect.
From www.slideserve.com
PPT Solvation Models PowerPoint Presentation, free download ID927911 Example Of Solvent Effect Polar protic solvents are capable of making hydrogen bonding i.e. Polar, protic solvents can stabilize ions through very strong intermolecular attractions. The protic hydrogen can strongly interact with anions, whereas. Solvent effects (how different solvents behave) and solvation (how solvents organize around a solute molecule) are very important to consider in thinking about acidity. According to this effect, the basicity. Example Of Solvent Effect.
From www.masterorganicchemistry.com
Deciding SN1/SN2/E1/E2 (3) The Solvent Master Organic Chemistry Example Of Solvent Effect They contain a hydrogen connected to an electronegative atom and thus can make intermolecular. Solvent effects (how different solvents behave) and solvation (how solvents organize around a solute molecule) are very important to consider in thinking about acidity. According to this effect, the basicity of the solvents. Solvent effect refers to the impact of the solvent on the properties and. Example Of Solvent Effect.
From www.chemistrysteps.com
The Role of Solvent in SN1, SN2, E1 and E2 Reactions Chemistry Steps Example Of Solvent Effect Polar protic solvents are capable of making hydrogen bonding i.e. They contain a hydrogen connected to an electronegative atom and thus can make intermolecular. Polar, protic solvents can stabilize ions through very strong intermolecular attractions. Solvent effects (how different solvents behave) and solvation (how solvents organize around a solute molecule) are very important to consider in thinking about acidity. The. Example Of Solvent Effect.
From www.slideserve.com
PPT Substitution and Elimination PowerPoint Presentation, free Example Of Solvent Effect Polar, protic solvents can stabilize ions through very strong intermolecular attractions. The leveling effect is the ability of a solvent to enhance the strength of weak acids or bases to that of strong ones. Solvent effects (how different solvents behave) and solvation (how solvents organize around a solute molecule) are very important to consider in thinking about acidity. Solvent effect. Example Of Solvent Effect.
From www.slideserve.com
PPT Acids & Bases/ Organic Chemistry PowerPoint Presentation ID1979697 Example Of Solvent Effect Solvent effect refers to the impact of the solvent on the properties and behavior of organic molecules, including their electronic structure. The protic hydrogen can strongly interact with anions, whereas. Solvent effects (how different solvents behave) and solvation (how solvents organize around a solute molecule) are very important to consider in thinking about acidity. Polar protic solvents are capable of. Example Of Solvent Effect.
From www.slideshare.net
Solvent Effects on Chemical Reaction PPT Example Of Solvent Effect Solvent effects (how different solvents behave) and solvation (how solvents organize around a solute molecule) are very important to consider in thinking about acidity. Polar, protic solvents can stabilize ions through very strong intermolecular attractions. According to this effect, the basicity of the solvents. Solvent effect refers to the impact of the solvent on the properties and behavior of organic. Example Of Solvent Effect.
From www.slideserve.com
PPT Chapter 14 PowerPoint Presentation, free download ID3622961 Example Of Solvent Effect The protic hydrogen can strongly interact with anions, whereas. Polar, protic solvents can stabilize ions through very strong intermolecular attractions. The leveling effect is the ability of a solvent to enhance the strength of weak acids or bases to that of strong ones. They contain a hydrogen connected to an electronegative atom and thus can make intermolecular. Solvent effects (how. Example Of Solvent Effect.