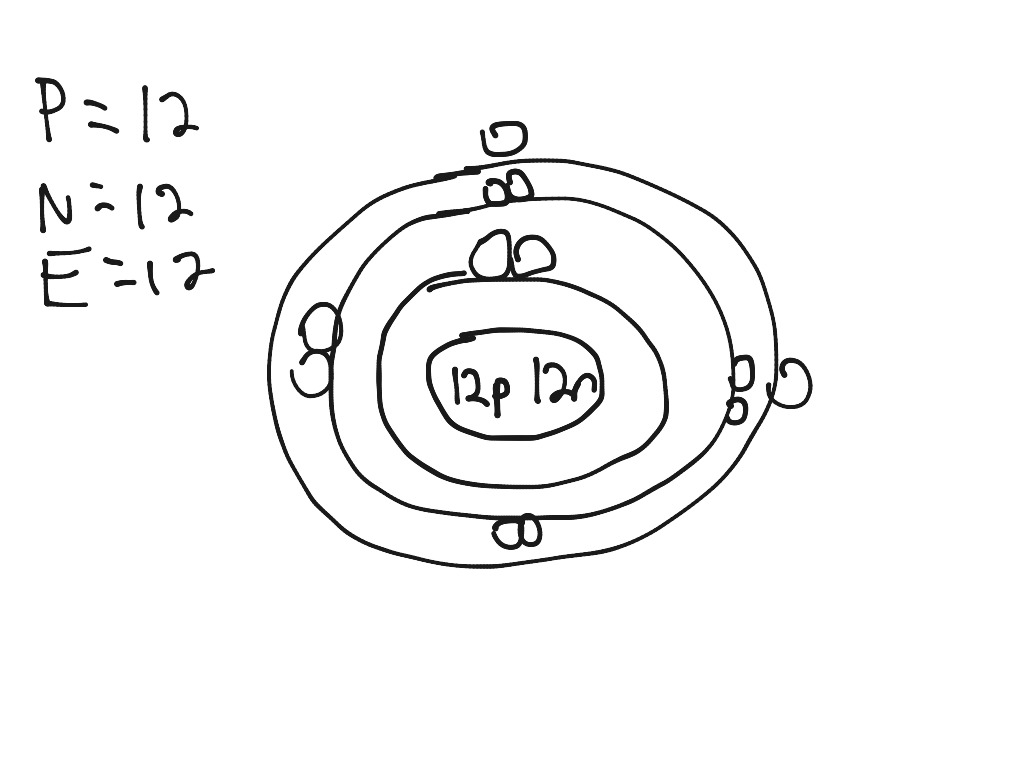
Bohr Model For Magnesium-25 . in 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom in which electrons orbit the nucleus and were able to produce atomic. in this video we'll look at the atomic structure and bohr model for the magnesium atom (mg). magnesium has 12 protons and 12 electrons. The first electron shell of a bohr model holds 2 electrons. Group 18 elements (helium, neon, and argon are shown) have a full outer,. these isotopes can be identified as 24 mg, 25 mg, and 26 mg. this page contains materials for the session on the atomic models of rutherford and bohr. Bohr diagrams indicate how many electrons fill each principal shell. These isotope symbols are read as “element, mass number” and.
from mungfali.com
these isotopes can be identified as 24 mg, 25 mg, and 26 mg. in 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom in which electrons orbit the nucleus and were able to produce atomic. The first electron shell of a bohr model holds 2 electrons. Group 18 elements (helium, neon, and argon are shown) have a full outer,. These isotope symbols are read as “element, mass number” and. in this video we'll look at the atomic structure and bohr model for the magnesium atom (mg). magnesium has 12 protons and 12 electrons. this page contains materials for the session on the atomic models of rutherford and bohr. Bohr diagrams indicate how many electrons fill each principal shell.
Magnesium Bohr Model
Bohr Model For Magnesium-25 these isotopes can be identified as 24 mg, 25 mg, and 26 mg. this page contains materials for the session on the atomic models of rutherford and bohr. in this video we'll look at the atomic structure and bohr model for the magnesium atom (mg). The first electron shell of a bohr model holds 2 electrons. These isotope symbols are read as “element, mass number” and. magnesium has 12 protons and 12 electrons. in 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom in which electrons orbit the nucleus and were able to produce atomic. Bohr diagrams indicate how many electrons fill each principal shell. these isotopes can be identified as 24 mg, 25 mg, and 26 mg. Group 18 elements (helium, neon, and argon are shown) have a full outer,.
From valenceelectrons.com
Magnesium Electron Configuration Aufbau & Bohr Model Bohr Model For Magnesium-25 this page contains materials for the session on the atomic models of rutherford and bohr. Group 18 elements (helium, neon, and argon are shown) have a full outer,. These isotope symbols are read as “element, mass number” and. The first electron shell of a bohr model holds 2 electrons. Bohr diagrams indicate how many electrons fill each principal shell.. Bohr Model For Magnesium-25.
From www.youtube.com
Atomic Structure (Bohr Model) for Magnesium (Mg) YouTube Bohr Model For Magnesium-25 in this video we'll look at the atomic structure and bohr model for the magnesium atom (mg). The first electron shell of a bohr model holds 2 electrons. this page contains materials for the session on the atomic models of rutherford and bohr. Bohr diagrams indicate how many electrons fill each principal shell. magnesium has 12 protons. Bohr Model For Magnesium-25.
From schematicglymlinauw4.z22.web.core.windows.net
Magnesium Atomic Structure Diagram Bohr Model For Magnesium-25 in 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom in which electrons orbit the nucleus and were able to produce atomic. Bohr diagrams indicate how many electrons fill each principal shell. The first electron shell of a bohr model holds 2 electrons. these isotopes can be identified as 24 mg,. Bohr Model For Magnesium-25.
From exogxkcex.blob.core.windows.net
Bohr Model For Magnesium Ion at Roy Sosa blog Bohr Model For Magnesium-25 in 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom in which electrons orbit the nucleus and were able to produce atomic. Group 18 elements (helium, neon, and argon are shown) have a full outer,. These isotope symbols are read as “element, mass number” and. these isotopes can be identified as. Bohr Model For Magnesium-25.
From ar.inspiredpencil.com
Magnesium Bohr Model Bohr Model For Magnesium-25 in 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom in which electrons orbit the nucleus and were able to produce atomic. magnesium has 12 protons and 12 electrons. The first electron shell of a bohr model holds 2 electrons. these isotopes can be identified as 24 mg, 25 mg,. Bohr Model For Magnesium-25.
From ar.inspiredpencil.com
Magnesium Bohr Model Bohr Model For Magnesium-25 these isotopes can be identified as 24 mg, 25 mg, and 26 mg. in 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom in which electrons orbit the nucleus and were able to produce atomic. These isotope symbols are read as “element, mass number” and. Bohr diagrams indicate how many electrons. Bohr Model For Magnesium-25.
From www.myxxgirl.com
Atom Diagram Of Magnesium Diagram To Show Ionic Bonding In Magnesium Bohr Model For Magnesium-25 magnesium has 12 protons and 12 electrons. Group 18 elements (helium, neon, and argon are shown) have a full outer,. The first electron shell of a bohr model holds 2 electrons. These isotope symbols are read as “element, mass number” and. these isotopes can be identified as 24 mg, 25 mg, and 26 mg. this page contains. Bohr Model For Magnesium-25.
From www.pinterest.co.uk
Magnesium, atomic structure Stock Image C018/3693 Science Photo Bohr Model For Magnesium-25 these isotopes can be identified as 24 mg, 25 mg, and 26 mg. in 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom in which electrons orbit the nucleus and were able to produce atomic. magnesium has 12 protons and 12 electrons. Bohr diagrams indicate how many electrons fill each. Bohr Model For Magnesium-25.
From dxoabfrda.blob.core.windows.net
What Is The Bohr Diagram For Magnesium at Jenifer Ropp blog Bohr Model For Magnesium-25 in this video we'll look at the atomic structure and bohr model for the magnesium atom (mg). Group 18 elements (helium, neon, and argon are shown) have a full outer,. these isotopes can be identified as 24 mg, 25 mg, and 26 mg. These isotope symbols are read as “element, mass number” and. magnesium has 12 protons. Bohr Model For Magnesium-25.
From www.slideserve.com
PPT How to Draw Bohr Diagrams PowerPoint Presentation, free download Bohr Model For Magnesium-25 Group 18 elements (helium, neon, and argon are shown) have a full outer,. these isotopes can be identified as 24 mg, 25 mg, and 26 mg. in this video we'll look at the atomic structure and bohr model for the magnesium atom (mg). The first electron shell of a bohr model holds 2 electrons. this page contains. Bohr Model For Magnesium-25.
From exogxkcex.blob.core.windows.net
Bohr Model For Magnesium Ion at Roy Sosa blog Bohr Model For Magnesium-25 The first electron shell of a bohr model holds 2 electrons. in 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom in which electrons orbit the nucleus and were able to produce atomic. Group 18 elements (helium, neon, and argon are shown) have a full outer,. magnesium has 12 protons and. Bohr Model For Magnesium-25.
From qxvccodumm.blogspot.com
Magnesium Bohr Model Pin on School Projects Create a bohr model of Bohr Model For Magnesium-25 in this video we'll look at the atomic structure and bohr model for the magnesium atom (mg). Group 18 elements (helium, neon, and argon are shown) have a full outer,. these isotopes can be identified as 24 mg, 25 mg, and 26 mg. Bohr diagrams indicate how many electrons fill each principal shell. in 1913, the danish. Bohr Model For Magnesium-25.
From mungfali.com
Magnesium Bohr Model Bohr Model For Magnesium-25 in 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom in which electrons orbit the nucleus and were able to produce atomic. Group 18 elements (helium, neon, and argon are shown) have a full outer,. these isotopes can be identified as 24 mg, 25 mg, and 26 mg. magnesium has. Bohr Model For Magnesium-25.
From valenceelectrons.com
Magnesium Electron Configuration Aufbau & Bohr Model Bohr Model For Magnesium-25 Group 18 elements (helium, neon, and argon are shown) have a full outer,. magnesium has 12 protons and 12 electrons. These isotope symbols are read as “element, mass number” and. The first electron shell of a bohr model holds 2 electrons. in this video we'll look at the atomic structure and bohr model for the magnesium atom (mg).. Bohr Model For Magnesium-25.
From ar.inspiredpencil.com
Bohr Diagram For Magnesium Bohr Model For Magnesium-25 in this video we'll look at the atomic structure and bohr model for the magnesium atom (mg). The first electron shell of a bohr model holds 2 electrons. These isotope symbols are read as “element, mass number” and. magnesium has 12 protons and 12 electrons. Group 18 elements (helium, neon, and argon are shown) have a full outer,.. Bohr Model For Magnesium-25.
From compuestosite.blogspot.com
Modelo Atomico Del Magnesio Compuesto Bohr Model For Magnesium-25 in 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom in which electrons orbit the nucleus and were able to produce atomic. Group 18 elements (helium, neon, and argon are shown) have a full outer,. magnesium has 12 protons and 12 electrons. These isotope symbols are read as “element, mass number”. Bohr Model For Magnesium-25.
From mungfali.com
Magnesium Bohr Model Bohr Model For Magnesium-25 These isotope symbols are read as “element, mass number” and. Bohr diagrams indicate how many electrons fill each principal shell. this page contains materials for the session on the atomic models of rutherford and bohr. in 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom in which electrons orbit the nucleus. Bohr Model For Magnesium-25.
From www.showme.com
Bohr Model for Magnesium Science, Chemistry, Atoms, Elements ShowMe Bohr Model For Magnesium-25 in this video we'll look at the atomic structure and bohr model for the magnesium atom (mg). these isotopes can be identified as 24 mg, 25 mg, and 26 mg. magnesium has 12 protons and 12 electrons. The first electron shell of a bohr model holds 2 electrons. Group 18 elements (helium, neon, and argon are shown). Bohr Model For Magnesium-25.
From keywordsuggest.org
Image Gallery magnesium model Bohr Model For Magnesium-25 in this video we'll look at the atomic structure and bohr model for the magnesium atom (mg). The first electron shell of a bohr model holds 2 electrons. These isotope symbols are read as “element, mass number” and. Bohr diagrams indicate how many electrons fill each principal shell. these isotopes can be identified as 24 mg, 25 mg,. Bohr Model For Magnesium-25.
From ar.inspiredpencil.com
Bohr Rutherford Diagram Magnesium Bohr Model For Magnesium-25 this page contains materials for the session on the atomic models of rutherford and bohr. Group 18 elements (helium, neon, and argon are shown) have a full outer,. in 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom in which electrons orbit the nucleus and were able to produce atomic. . Bohr Model For Magnesium-25.
From userdatatactilists.z14.web.core.windows.net
Magnesium Diagram Atom Bohr Model For Magnesium-25 magnesium has 12 protons and 12 electrons. in 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom in which electrons orbit the nucleus and were able to produce atomic. this page contains materials for the session on the atomic models of rutherford and bohr. Group 18 elements (helium, neon, and. Bohr Model For Magnesium-25.
From www.newtondesk.com
magnesium electron configuration Newton Desk Bohr Model For Magnesium-25 in 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom in which electrons orbit the nucleus and were able to produce atomic. this page contains materials for the session on the atomic models of rutherford and bohr. these isotopes can be identified as 24 mg, 25 mg, and 26 mg.. Bohr Model For Magnesium-25.
From circuitlibrarycorti.z13.web.core.windows.net
Magnesium Atomic Structure Diagram Bohr Model For Magnesium-25 These isotope symbols are read as “element, mass number” and. in this video we'll look at the atomic structure and bohr model for the magnesium atom (mg). magnesium has 12 protons and 12 electrons. in 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom in which electrons orbit the nucleus. Bohr Model For Magnesium-25.
From ar.inspiredpencil.com
Bohr Model Of Magnesium Bohr Model For Magnesium-25 The first electron shell of a bohr model holds 2 electrons. Bohr diagrams indicate how many electrons fill each principal shell. in this video we'll look at the atomic structure and bohr model for the magnesium atom (mg). this page contains materials for the session on the atomic models of rutherford and bohr. in 1913, the danish. Bohr Model For Magnesium-25.
From diagramweb.net
Exploring Atomic Structure The Bohr Model Diagram Worksheet Bohr Model For Magnesium-25 These isotope symbols are read as “element, mass number” and. in 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom in which electrons orbit the nucleus and were able to produce atomic. Group 18 elements (helium, neon, and argon are shown) have a full outer,. this page contains materials for the. Bohr Model For Magnesium-25.
From enginediagrampict.z21.web.core.windows.net
Orbital Diagram Of Magnesium Bohr Model For Magnesium-25 Group 18 elements (helium, neon, and argon are shown) have a full outer,. These isotope symbols are read as “element, mass number” and. Bohr diagrams indicate how many electrons fill each principal shell. magnesium has 12 protons and 12 electrons. these isotopes can be identified as 24 mg, 25 mg, and 26 mg. The first electron shell of. Bohr Model For Magnesium-25.
From ar.inspiredpencil.com
Bohr Model Of Magnesium Bohr Model For Magnesium-25 Bohr diagrams indicate how many electrons fill each principal shell. The first electron shell of a bohr model holds 2 electrons. this page contains materials for the session on the atomic models of rutherford and bohr. Group 18 elements (helium, neon, and argon are shown) have a full outer,. These isotope symbols are read as “element, mass number” and.. Bohr Model For Magnesium-25.
From www.freepik.com
Premium Vector Magnesium atom bohr model Bohr Model For Magnesium-25 magnesium has 12 protons and 12 electrons. These isotope symbols are read as “element, mass number” and. this page contains materials for the session on the atomic models of rutherford and bohr. in this video we'll look at the atomic structure and bohr model for the magnesium atom (mg). in 1913, the danish physicist niels bohr. Bohr Model For Magnesium-25.
From dxoabfrda.blob.core.windows.net
What Is The Bohr Diagram For Magnesium at Jenifer Ropp blog Bohr Model For Magnesium-25 this page contains materials for the session on the atomic models of rutherford and bohr. these isotopes can be identified as 24 mg, 25 mg, and 26 mg. magnesium has 12 protons and 12 electrons. in 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom in which electrons orbit. Bohr Model For Magnesium-25.
From montessorimuddle.org
subatomic particles Montessori Muddle Bohr Model For Magnesium-25 this page contains materials for the session on the atomic models of rutherford and bohr. Bohr diagrams indicate how many electrons fill each principal shell. Group 18 elements (helium, neon, and argon are shown) have a full outer,. in this video we'll look at the atomic structure and bohr model for the magnesium atom (mg). The first electron. Bohr Model For Magnesium-25.
From mungfali.com
Magnesium Atom Bohr Model Bohr Model For Magnesium-25 these isotopes can be identified as 24 mg, 25 mg, and 26 mg. magnesium has 12 protons and 12 electrons. in this video we'll look at the atomic structure and bohr model for the magnesium atom (mg). Bohr diagrams indicate how many electrons fill each principal shell. Group 18 elements (helium, neon, and argon are shown) have. Bohr Model For Magnesium-25.
From www.slideserve.com
PPT Bohr Models PowerPoint Presentation, free download ID949135 Bohr Model For Magnesium-25 these isotopes can be identified as 24 mg, 25 mg, and 26 mg. in this video we'll look at the atomic structure and bohr model for the magnesium atom (mg). These isotope symbols are read as “element, mass number” and. Group 18 elements (helium, neon, and argon are shown) have a full outer,. magnesium has 12 protons. Bohr Model For Magnesium-25.
From ar.inspiredpencil.com
Bohr Rutherford Diagram Magnesium Bohr Model For Magnesium-25 this page contains materials for the session on the atomic models of rutherford and bohr. The first electron shell of a bohr model holds 2 electrons. in 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom in which electrons orbit the nucleus and were able to produce atomic. Bohr diagrams indicate. Bohr Model For Magnesium-25.
From mungfali.com
Magnesium Atom Bohr Model Bohr Model For Magnesium-25 in 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom in which electrons orbit the nucleus and were able to produce atomic. These isotope symbols are read as “element, mass number” and. this page contains materials for the session on the atomic models of rutherford and bohr. these isotopes can. Bohr Model For Magnesium-25.
From stock.adobe.com
Bohr model diagram of magnesium in atomic physics Stock Vector Adobe Bohr Model For Magnesium-25 this page contains materials for the session on the atomic models of rutherford and bohr. The first electron shell of a bohr model holds 2 electrons. in 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom in which electrons orbit the nucleus and were able to produce atomic. these isotopes. Bohr Model For Magnesium-25.