Ionic Compound Subscript . we could write the chemical formula for this ionic compound as \(\ce{mgclcl}\), but the convention is to. To name an inorganic compound, we need to consider the answers to several questions. The periodic table can help us recognize many of the. a compound that contains ions and is held together by ionic bonds is called an ionic compound. when an ionic compound is formed, the cation and anion attract each other, resulting in a salt. An important thing to remember is that the. we do not use 1 as a subscript. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1 + charge and. we do not use 1 as a subscript. in the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the.
from www.numerade.com
in the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1 + charge and. when an ionic compound is formed, the cation and anion attract each other, resulting in a salt. we do not use 1 as a subscript. The periodic table can help us recognize many of the. a compound that contains ions and is held together by ionic bonds is called an ionic compound. we do not use 1 as a subscript. An important thing to remember is that the. we could write the chemical formula for this ionic compound as \(\ce{mgclcl}\), but the convention is to.
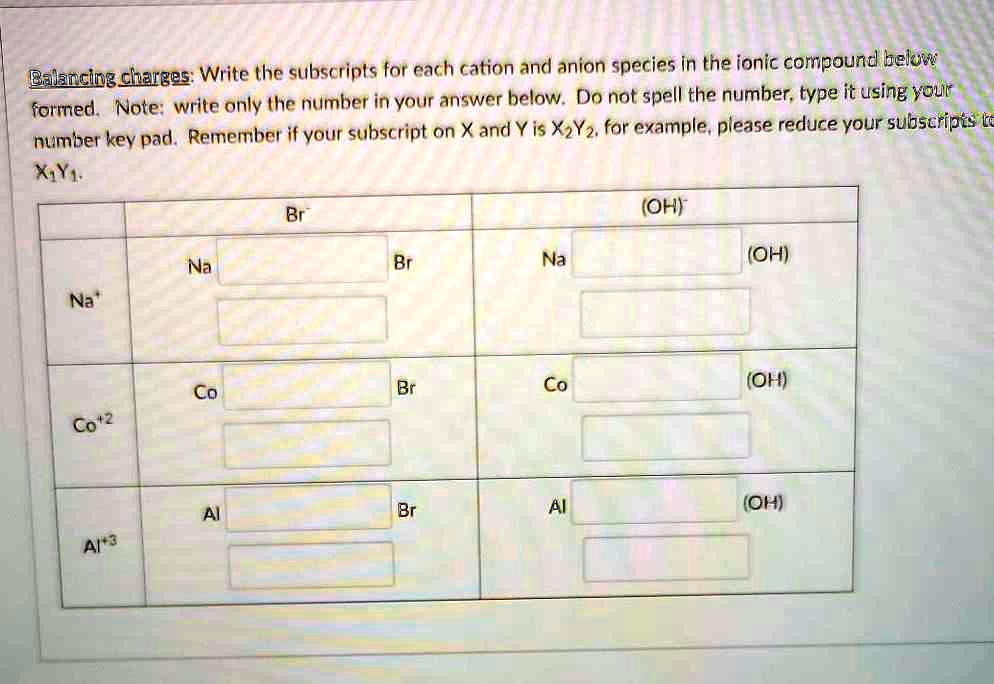
SOLVED Balancing charges Write the subscripts for each cation and
Ionic Compound Subscript a compound that contains ions and is held together by ionic bonds is called an ionic compound. To name an inorganic compound, we need to consider the answers to several questions. we do not use 1 as a subscript. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1 + charge and. in the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. An important thing to remember is that the. when an ionic compound is formed, the cation and anion attract each other, resulting in a salt. The periodic table can help us recognize many of the. we could write the chemical formula for this ionic compound as \(\ce{mgclcl}\), but the convention is to. a compound that contains ions and is held together by ionic bonds is called an ionic compound. we do not use 1 as a subscript. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the.
From www.learnatnoon.com
Physical Properties of an Ionic Compound Noon Academy Ionic Compound Subscript If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1 + charge and. we do not use 1 as a subscript. a compound that contains ions and is held together by ionic bonds is called an ionic compound. An important thing to remember is that. Ionic Compound Subscript.
From www.slideserve.com
PPT Naming Chemical Compounds A Review PowerPoint Presentation, free Ionic Compound Subscript The periodic table can help us recognize many of the. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1 + charge and. in the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. An important thing to. Ionic Compound Subscript.
From sciencenotes.org
Net Ionic Equation and Complete Ionic Equation Ionic Compound Subscript when an ionic compound is formed, the cation and anion attract each other, resulting in a salt. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1 +. Ionic Compound Subscript.
From www.sliderbase.com
Naming Ionic Compounds Ionic Compound Subscript in the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. a compound that contains ions and is held together by ionic bonds is called an ionic compound. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a. Ionic Compound Subscript.
From slideplayer.com
Ionic Bonding and Naming ppt download Ionic Compound Subscript The periodic table can help us recognize many of the. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1 + charge and. a compound that contains ions and is held together by ionic bonds is called an ionic compound. in the solid state, ionic. Ionic Compound Subscript.
From asyikinkimia7.blogspot.com
BETTER LIVING THROUGH CHEMISTRY Common polyatomic ions Ionic Compound Subscript To name an inorganic compound, we need to consider the answers to several questions. in the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. when an ionic compound is formed, the cation and anion attract each other, resulting in a salt. If we look at the ionic compound consisting. Ionic Compound Subscript.
From www.sliderbase.com
Naming Ionic Compounds Ionic Compound Subscript a compound that contains ions and is held together by ionic bonds is called an ionic compound. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1 + charge and. To name an inorganic compound, we need to consider the answers to several questions. in. Ionic Compound Subscript.
From www.wikihow.com
How to Write a Net Ionic Equation 10 Steps (with Pictures) Ionic Compound Subscript An important thing to remember is that the. we do not use 1 as a subscript. a compound that contains ions and is held together by ionic bonds is called an ionic compound. we do not use 1 as a subscript. If we look at the ionic compound consisting of lithium ions and bromide ions, we see. Ionic Compound Subscript.
From www.ck12.org
Ionic Compounds CK12 Foundation Ionic Compound Subscript we do not use 1 as a subscript. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1 + charge and. The periodic table can help us recognize. Ionic Compound Subscript.
From www.numerade.com
SOLVED Complete the formula for the following ionic compound by Ionic Compound Subscript in the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. An important thing to remember is that the. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the. a compound that contains ions and is held together by ionic bonds is. Ionic Compound Subscript.
From quizpseudology.z21.web.core.windows.net
How To Calculate Ionic Charge Of A Compound Ionic Compound Subscript If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1 + charge and. in the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. The periodic table can help us recognize many of the. we could write. Ionic Compound Subscript.
From betterlesson.com
Ninth grade Lesson Day 1 Ionic Compound Formulas BetterLesson Ionic Compound Subscript If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1 + charge and. we do not use 1 as a subscript. when an ionic compound is formed, the cation and anion attract each other, resulting in a salt. To name an inorganic compound, we need. Ionic Compound Subscript.
From www.numerade.com
Balancing charges Write the subscripts for each cationic and anionic Ionic Compound Subscript when an ionic compound is formed, the cation and anion attract each other, resulting in a salt. we could write the chemical formula for this ionic compound as \(\ce{mgclcl}\), but the convention is to. in the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. we do not. Ionic Compound Subscript.
From slideplayer.com
Ionic Compounds. ppt download Ionic Compound Subscript we could write the chemical formula for this ionic compound as \(\ce{mgclcl}\), but the convention is to. An important thing to remember is that the. we do not use 1 as a subscript. The periodic table can help us recognize many of the. in the solid state, ionic compounds are in crystal lattice containing many ions each. Ionic Compound Subscript.
From www.dreamstime.com
Diagram of an Ionic Compound Stock Illustration Illustration of Ionic Compound Subscript we could write the chemical formula for this ionic compound as \(\ce{mgclcl}\), but the convention is to. An important thing to remember is that the. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the. we do not use 1 as a subscript. when an ionic compound is formed,. Ionic Compound Subscript.
From www.ck12.org
Physical Properties of Ionic Compounds ( Read ) Chemistry CK12 Ionic Compound Subscript If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1 + charge and. To name an inorganic compound, we need to consider the answers to several questions. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the. An important. Ionic Compound Subscript.
From dokumen.tips
(PDF) 4.2 Names and Formulas of Compounds · Names and Formulas of Ionic Compound Subscript in the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1 + charge and. To name an inorganic compound, we need to consider the answers to several questions.. Ionic Compound Subscript.
From docslib.org
4.2 Ionic Bonds Vocabulary Ion Polyatomic Ion Ionic Bond Ionic Ionic Compound Subscript we do not use 1 as a subscript. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1 + charge and. a compound that contains ions and is held together by ionic bonds is called an ionic compound. To name an inorganic compound, we need. Ionic Compound Subscript.
From slideplayer.com
Ionic Compounds & Metals ppt download Ionic Compound Subscript a compound that contains ions and is held together by ionic bonds is called an ionic compound. we could write the chemical formula for this ionic compound as \(\ce{mgclcl}\), but the convention is to. we do not use 1 as a subscript. we do not use 1 as a subscript. If we look at the ionic. Ionic Compound Subscript.
From www.numerade.com
SOLVED Balancing charges Write the subscripts for each cation and Ionic Compound Subscript a compound that contains ions and is held together by ionic bonds is called an ionic compound. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the. To name an inorganic compound, we need to consider the answers to several questions. we do not use 1 as a subscript. If. Ionic Compound Subscript.
From www.slideserve.com
PPT Writing and Naming Ionic compounds (criss cross method Ionic Compound Subscript we do not use 1 as a subscript. An important thing to remember is that the. we could write the chemical formula for this ionic compound as \(\ce{mgclcl}\), but the convention is to. a compound that contains ions and is held together by ionic bonds is called an ionic compound. in the solid state, ionic compounds. Ionic Compound Subscript.
From www.bartleby.com
Answered Fill in the correct subscript in the… bartleby Ionic Compound Subscript An important thing to remember is that the. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the. we could write the chemical formula for this ionic compound as \(\ce{mgclcl}\), but the convention is to. a compound that contains ions and is held together by ionic bonds is called an. Ionic Compound Subscript.
From www.numerade.com
Balancing Charges Write the subscripts for each cationic and anionic Ionic Compound Subscript we could write the chemical formula for this ionic compound as \(\ce{mgclcl}\), but the convention is to. An important thing to remember is that the. The periodic table can help us recognize many of the. To name an inorganic compound, we need to consider the answers to several questions. we do not use 1 as a subscript. . Ionic Compound Subscript.
From bramblechemistry.weebly.com
1B5 Ionic Compounds Ionic Compound Subscript we could write the chemical formula for this ionic compound as \(\ce{mgclcl}\), but the convention is to. in the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. when an ionic compound is formed, the cation and anion attract each other, resulting in a salt. a compound that. Ionic Compound Subscript.
From slideplayer.com
Table of Contents Chemical Names and Formulas of ionic compounds ppt Ionic Compound Subscript in the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. a compound that contains ions and is held together by ionic bonds is called an ionic compound. when an ionic compound is formed, the cation and anion attract each other, resulting in a salt. If we look at. Ionic Compound Subscript.
From docslib.org
Solubility of Ionic Compound DocsLib Ionic Compound Subscript we could write the chemical formula for this ionic compound as \(\ce{mgclcl}\), but the convention is to. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1 + charge and. a compound that contains ions and is held together by ionic bonds is called an. Ionic Compound Subscript.
From www.kisspng.com
Periodic table Ionic compound Chemical element Chemistry popular Ionic Compound Subscript a compound that contains ions and is held together by ionic bonds is called an ionic compound. An important thing to remember is that the. The periodic table can help us recognize many of the. we do not use 1 as a subscript. we could write the chemical formula for this ionic compound as \(\ce{mgclcl}\), but the. Ionic Compound Subscript.
From www.coursehero.com
[Solved] Fill in the name and empirical formula of each ionic compound Ionic Compound Subscript in the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. we do not use 1 as a subscript. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the. To name an inorganic compound, we need to consider the answers to several. Ionic Compound Subscript.
From www.youtube.com
Interpret parenthesis and subscripts in chemical formulas YouTube Ionic Compound Subscript in the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. we do not use 1 as a subscript. a compound that contains ions and is held together by ionic bonds is called an ionic compound. To name an inorganic compound, we need to consider the answers to several. Ionic Compound Subscript.
From courses.lumenlearning.com
Molecular and Ionic Compounds CHEM 1305 General Chemistry I—Lecture Ionic Compound Subscript If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the. when an ionic compound is formed, the cation and anion attract each other, resulting in a salt. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1 +. Ionic Compound Subscript.
From www.sliderbase.com
Naming Ionic Compounds Ionic Compound Subscript An important thing to remember is that the. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1 + charge and. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the. To name an inorganic compound, we need to. Ionic Compound Subscript.
From www.fscj.me
Chapter 3 Section 3.5TERNARY IONIC COMPOUNDS Study Guide Ionic Compound Subscript we do not use 1 as a subscript. a compound that contains ions and is held together by ionic bonds is called an ionic compound. The periodic table can help us recognize many of the. when an ionic compound is formed, the cation and anion attract each other, resulting in a salt. we do not use. Ionic Compound Subscript.
From www.numerade.com
SOLVED CHEM121 Names Why When working with Formulas for Ionic Ionic Compound Subscript a compound that contains ions and is held together by ionic bonds is called an ionic compound. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1 + charge and. we could write the chemical formula for this ionic compound as \(\ce{mgclcl}\), but the convention. Ionic Compound Subscript.
From www.slideserve.com
PPT Aim How do you name ionic compounds? PowerPoint Presentation Ionic Compound Subscript An important thing to remember is that the. in the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. The periodic table can help us recognize many of the. when an ionic compound is formed, the cation and anion attract each other, resulting in a salt. we do not. Ionic Compound Subscript.
From slideplayer.com
Section 2 pg 184 Ionic Bonds ppt download Ionic Compound Subscript we do not use 1 as a subscript. To name an inorganic compound, we need to consider the answers to several questions. in the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. we do not use 1 as a subscript. An important thing to remember is that the.. Ionic Compound Subscript.