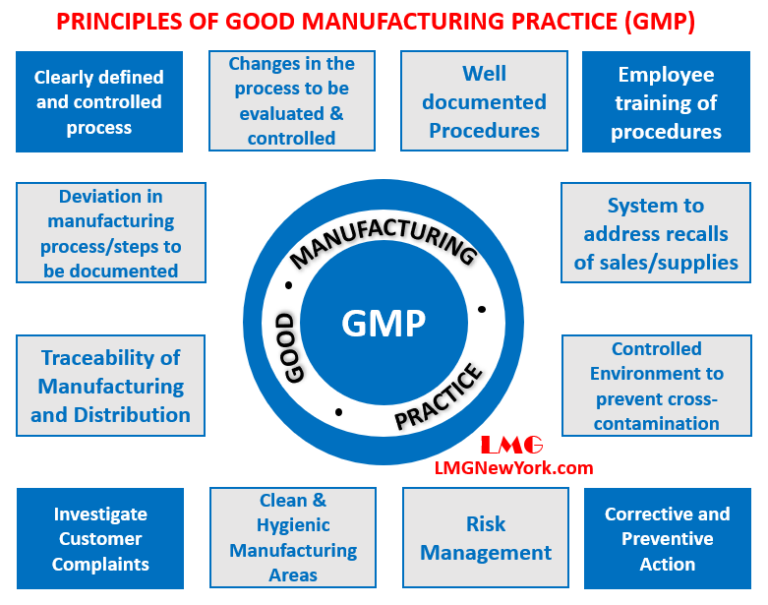
Materials Management Gmp . It is designed to minimize the. Material portability, using standardized materials, processes and products across facilities and geographic boundaries, reduces the risk of variable quality and supply. We focused on elements in ich q7 where further explanation and/or clarification are useful. The european medicines agency's (ema) provides answers to frequently asked questions on good manufacturing practice (gmp) and good. Training in current good manufacturing practice shall be conducted by qualified individuals on a continuing basis and with sufficient frequency. In this guide “manufacturing” is defined to include all operations of receipt of materials, production, packaging, repackaging, labelling, relabelling,. Good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of quality assurance. Good manufacturing practice (gmp) is a system for ensuring that products are consistently produced and controlled according to quality standards. Ich q11 defines what to file as ‘api staring.
from lmgnewyork.com
Ich q11 defines what to file as ‘api staring. It is designed to minimize the. Good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of quality assurance. We focused on elements in ich q7 where further explanation and/or clarification are useful. The european medicines agency's (ema) provides answers to frequently asked questions on good manufacturing practice (gmp) and good. Good manufacturing practice (gmp) is a system for ensuring that products are consistently produced and controlled according to quality standards. In this guide “manufacturing” is defined to include all operations of receipt of materials, production, packaging, repackaging, labelling, relabelling,. Material portability, using standardized materials, processes and products across facilities and geographic boundaries, reduces the risk of variable quality and supply. Training in current good manufacturing practice shall be conducted by qualified individuals on a continuing basis and with sufficient frequency.
GMP Good Manufacturing Practice LMG New York
Materials Management Gmp Good manufacturing practice (gmp) is a system for ensuring that products are consistently produced and controlled according to quality standards. Good manufacturing practice (gmp) is a system for ensuring that products are consistently produced and controlled according to quality standards. Material portability, using standardized materials, processes and products across facilities and geographic boundaries, reduces the risk of variable quality and supply. Good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of quality assurance. We focused on elements in ich q7 where further explanation and/or clarification are useful. Ich q11 defines what to file as ‘api staring. In this guide “manufacturing” is defined to include all operations of receipt of materials, production, packaging, repackaging, labelling, relabelling,. Training in current good manufacturing practice shall be conducted by qualified individuals on a continuing basis and with sufficient frequency. It is designed to minimize the. The european medicines agency's (ema) provides answers to frequently asked questions on good manufacturing practice (gmp) and good.
From www.slideserve.com
PPT Good Manufacturing Practices Purpose and Principles of GMP Materials Management Gmp We focused on elements in ich q7 where further explanation and/or clarification are useful. Good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of quality assurance. The european medicines agency's (ema) provides answers to frequently asked questions on good manufacturing practice (gmp) and good. Good manufacturing practice (gmp) is a system for. Materials Management Gmp.
From www.slideserve.com
PPT Basic Principles of GMP PowerPoint Presentation, free download Materials Management Gmp Material portability, using standardized materials, processes and products across facilities and geographic boundaries, reduces the risk of variable quality and supply. Training in current good manufacturing practice shall be conducted by qualified individuals on a continuing basis and with sufficient frequency. It is designed to minimize the. We focused on elements in ich q7 where further explanation and/or clarification are. Materials Management Gmp.
From www.youtube.com
Difference between GMP (Good Manufacturing Practices)🏭 & GLP (Good Materials Management Gmp Good manufacturing practice (gmp) is a system for ensuring that products are consistently produced and controlled according to quality standards. Good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of quality assurance. The european medicines agency's (ema) provides answers to frequently asked questions on good manufacturing practice (gmp) and good. In this. Materials Management Gmp.
From solutionpharmacy.in
GMP Requirements Solution Parmacy Materials Management Gmp In this guide “manufacturing” is defined to include all operations of receipt of materials, production, packaging, repackaging, labelling, relabelling,. The european medicines agency's (ema) provides answers to frequently asked questions on good manufacturing practice (gmp) and good. Good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of quality assurance. Good manufacturing practice. Materials Management Gmp.
From www.bulblaboratories.com
Our First GMP Facility Design Good Manufacturing Practice Materials Management Gmp Good manufacturing practice (gmp) is a system for ensuring that products are consistently produced and controlled according to quality standards. Ich q11 defines what to file as ‘api staring. The european medicines agency's (ema) provides answers to frequently asked questions on good manufacturing practice (gmp) and good. Training in current good manufacturing practice shall be conducted by qualified individuals on. Materials Management Gmp.
From www.researchandmarkets.com
GMP Raw Materials Program Risk Management Research and Markets Materials Management Gmp It is designed to minimize the. We focused on elements in ich q7 where further explanation and/or clarification are useful. Good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of quality assurance. In this guide “manufacturing” is defined to include all operations of receipt of materials, production, packaging, repackaging, labelling, relabelling,. Ich. Materials Management Gmp.
From www.slideteam.net
Pharmaceutical Quality Control Framework With GMP Compliance PPT Example Materials Management Gmp Material portability, using standardized materials, processes and products across facilities and geographic boundaries, reduces the risk of variable quality and supply. We focused on elements in ich q7 where further explanation and/or clarification are useful. Training in current good manufacturing practice shall be conducted by qualified individuals on a continuing basis and with sufficient frequency. Ich q11 defines what to. Materials Management Gmp.
From www.slideserve.com
PPT Basic Principles of GMP PowerPoint Presentation, free download Materials Management Gmp Good manufacturing practice (gmp) is a system for ensuring that products are consistently produced and controlled according to quality standards. Good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of quality assurance. Material portability, using standardized materials, processes and products across facilities and geographic boundaries, reduces the risk of variable quality and. Materials Management Gmp.
From qmciso.org
GMP Good Manufacturing Practice Quality Management Certification Materials Management Gmp It is designed to minimize the. The european medicines agency's (ema) provides answers to frequently asked questions on good manufacturing practice (gmp) and good. Training in current good manufacturing practice shall be conducted by qualified individuals on a continuing basis and with sufficient frequency. Material portability, using standardized materials, processes and products across facilities and geographic boundaries, reduces the risk. Materials Management Gmp.
From www.slideshare.net
GMP Introduction Materials Management Gmp Training in current good manufacturing practice shall be conducted by qualified individuals on a continuing basis and with sufficient frequency. Good manufacturing practice (gmp) is a system for ensuring that products are consistently produced and controlled according to quality standards. Material portability, using standardized materials, processes and products across facilities and geographic boundaries, reduces the risk of variable quality and. Materials Management Gmp.
From www.slideserve.com
PPT BRC/ IoP Global Standard for Packaging and Packaging Materials Materials Management Gmp Good manufacturing practice (gmp) is a system for ensuring that products are consistently produced and controlled according to quality standards. In this guide “manufacturing” is defined to include all operations of receipt of materials, production, packaging, repackaging, labelling, relabelling,. Good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of quality assurance. Ich. Materials Management Gmp.
From www.slideserve.com
PPT Basic Principles of GMP PowerPoint Presentation, free download Materials Management Gmp It is designed to minimize the. Good manufacturing practice (gmp) is a system for ensuring that products are consistently produced and controlled according to quality standards. Material portability, using standardized materials, processes and products across facilities and geographic boundaries, reduces the risk of variable quality and supply. Ich q11 defines what to file as ‘api staring. Good manufacturing practices (gmp,. Materials Management Gmp.
From www.youtube.com
What are GMP Guidelines? Good Manufacturing Practices for Food Safety Materials Management Gmp Ich q11 defines what to file as ‘api staring. In this guide “manufacturing” is defined to include all operations of receipt of materials, production, packaging, repackaging, labelling, relabelling,. Material portability, using standardized materials, processes and products across facilities and geographic boundaries, reduces the risk of variable quality and supply. Training in current good manufacturing practice shall be conducted by qualified. Materials Management Gmp.
From www.slideserve.com
PPT Good Manufacturing Practices Purpose and Principles of GMP Materials Management Gmp Good manufacturing practice (gmp) is a system for ensuring that products are consistently produced and controlled according to quality standards. Ich q11 defines what to file as ‘api staring. Training in current good manufacturing practice shall be conducted by qualified individuals on a continuing basis and with sufficient frequency. The european medicines agency's (ema) provides answers to frequently asked questions. Materials Management Gmp.
From www.slideserve.com
PPT Basic Principles of GMP PowerPoint Presentation, free download Materials Management Gmp Good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of quality assurance. Ich q11 defines what to file as ‘api staring. The european medicines agency's (ema) provides answers to frequently asked questions on good manufacturing practice (gmp) and good. Training in current good manufacturing practice shall be conducted by qualified individuals on. Materials Management Gmp.
From www.pinterest.com
Best Practices For GMP Custom Manufacturing Services Custom Materials Management Gmp In this guide “manufacturing” is defined to include all operations of receipt of materials, production, packaging, repackaging, labelling, relabelling,. Training in current good manufacturing practice shall be conducted by qualified individuals on a continuing basis and with sufficient frequency. Good manufacturing practice (gmp) is a system for ensuring that products are consistently produced and controlled according to quality standards. Good. Materials Management Gmp.
From instantgmp.com
GMP Inventory Management Software InstantGMP™ INV Materials Management Gmp Ich q11 defines what to file as ‘api staring. Good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of quality assurance. It is designed to minimize the. In this guide “manufacturing” is defined to include all operations of receipt of materials, production, packaging, repackaging, labelling, relabelling,. Material portability, using standardized materials, processes. Materials Management Gmp.
From www.slideserve.com
PPT Good Manufacturing Practices PowerPoint Presentation ID364179 Materials Management Gmp Ich q11 defines what to file as ‘api staring. In this guide “manufacturing” is defined to include all operations of receipt of materials, production, packaging, repackaging, labelling, relabelling,. We focused on elements in ich q7 where further explanation and/or clarification are useful. Training in current good manufacturing practice shall be conducted by qualified individuals on a continuing basis and with. Materials Management Gmp.
From www.slideserve.com
PPT Good Manufacturing Practices Purpose and Principles of GMP Materials Management Gmp Good manufacturing practice (gmp) is a system for ensuring that products are consistently produced and controlled according to quality standards. Good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of quality assurance. Training in current good manufacturing practice shall be conducted by qualified individuals on a continuing basis and with sufficient frequency.. Materials Management Gmp.
From www.slideserve.com
PPT BRC/ IoP Global Standard for Packaging and Packaging Materials Materials Management Gmp In this guide “manufacturing” is defined to include all operations of receipt of materials, production, packaging, repackaging, labelling, relabelling,. Good manufacturing practice (gmp) is a system for ensuring that products are consistently produced and controlled according to quality standards. The european medicines agency's (ema) provides answers to frequently asked questions on good manufacturing practice (gmp) and good. It is designed. Materials Management Gmp.
From www.slideserve.com
PPT GOOD MANUFACTURING PRACTICES (GMP) PowerPoint Presentation, free Materials Management Gmp Training in current good manufacturing practice shall be conducted by qualified individuals on a continuing basis and with sufficient frequency. Ich q11 defines what to file as ‘api staring. Material portability, using standardized materials, processes and products across facilities and geographic boundaries, reduces the risk of variable quality and supply. Good manufacturing practice (gmp) is a system for ensuring that. Materials Management Gmp.
From www.researchgate.net
Schematic representation of the 2step GMP manufacturing process. (A Materials Management Gmp It is designed to minimize the. Ich q11 defines what to file as ‘api staring. The european medicines agency's (ema) provides answers to frequently asked questions on good manufacturing practice (gmp) and good. Good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of quality assurance. Good manufacturing practice (gmp) is a system. Materials Management Gmp.
From slcontrols.com
How Good Distribution Practice (GDP) differs from Good Manufacturing Materials Management Gmp Good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of quality assurance. Training in current good manufacturing practice shall be conducted by qualified individuals on a continuing basis and with sufficient frequency. The european medicines agency's (ema) provides answers to frequently asked questions on good manufacturing practice (gmp) and good. In this. Materials Management Gmp.
From lmgnewyork.com
GMP Good Manufacturing Practice LMG New York Materials Management Gmp Good manufacturing practice (gmp) is a system for ensuring that products are consistently produced and controlled according to quality standards. In this guide “manufacturing” is defined to include all operations of receipt of materials, production, packaging, repackaging, labelling, relabelling,. We focused on elements in ich q7 where further explanation and/or clarification are useful. It is designed to minimize the. Good. Materials Management Gmp.
From www.slideserve.com
PPT BRC/ IoP Global Standard for Packaging and Packaging Materials Materials Management Gmp We focused on elements in ich q7 where further explanation and/or clarification are useful. Good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of quality assurance. In this guide “manufacturing” is defined to include all operations of receipt of materials, production, packaging, repackaging, labelling, relabelling,. Good manufacturing practice (gmp) is a system. Materials Management Gmp.
From www.scilife.io
What are the 5 Main Components of GMP? Full definition Scilife Materials Management Gmp It is designed to minimize the. Ich q11 defines what to file as ‘api staring. Good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of quality assurance. In this guide “manufacturing” is defined to include all operations of receipt of materials, production, packaging, repackaging, labelling, relabelling,. Good manufacturing practice (gmp) is a. Materials Management Gmp.
From www.slideshare.net
GMP Manufacturing for Worldwide Clinical Trials Materials Management Gmp Good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of quality assurance. Material portability, using standardized materials, processes and products across facilities and geographic boundaries, reduces the risk of variable quality and supply. The european medicines agency's (ema) provides answers to frequently asked questions on good manufacturing practice (gmp) and good. Good. Materials Management Gmp.
From www.presentationeze.com
GMP Training Powerpoint Information & Training presentations Materials Management Gmp The european medicines agency's (ema) provides answers to frequently asked questions on good manufacturing practice (gmp) and good. Ich q11 defines what to file as ‘api staring. In this guide “manufacturing” is defined to include all operations of receipt of materials, production, packaging, repackaging, labelling, relabelling,. Training in current good manufacturing practice shall be conducted by qualified individuals on a. Materials Management Gmp.
From www.katsura-chemical.co.jp
Comply with GMP conditions for manufacturing safe and effective APIs Materials Management Gmp Good manufacturing practice (gmp) is a system for ensuring that products are consistently produced and controlled according to quality standards. We focused on elements in ich q7 where further explanation and/or clarification are useful. Ich q11 defines what to file as ‘api staring. It is designed to minimize the. In this guide “manufacturing” is defined to include all operations of. Materials Management Gmp.
From mpl.loesungsfabrik.de
What is GMP? Materials Management Gmp The european medicines agency's (ema) provides answers to frequently asked questions on good manufacturing practice (gmp) and good. In this guide “manufacturing” is defined to include all operations of receipt of materials, production, packaging, repackaging, labelling, relabelling,. Ich q11 defines what to file as ‘api staring. Good manufacturing practice (gmp) is a system for ensuring that products are consistently produced. Materials Management Gmp.
From www.researchandmarkets.com
Risk Management of Raw Materials in a GMP Environment inar inar Materials Management Gmp We focused on elements in ich q7 where further explanation and/or clarification are useful. Training in current good manufacturing practice shall be conducted by qualified individuals on a continuing basis and with sufficient frequency. It is designed to minimize the. In this guide “manufacturing” is defined to include all operations of receipt of materials, production, packaging, repackaging, labelling, relabelling,. Material. Materials Management Gmp.
From www.gmpsop.com
How to Control Packaging Materials in GMP Materials Management Gmp Training in current good manufacturing practice shall be conducted by qualified individuals on a continuing basis and with sufficient frequency. Ich q11 defines what to file as ‘api staring. We focused on elements in ich q7 where further explanation and/or clarification are useful. Material portability, using standardized materials, processes and products across facilities and geographic boundaries, reduces the risk of. Materials Management Gmp.
From mcsost-jur.blogspot.com
MEJORA CONTINUA & SOSTENIBILIDAD GMP&continuous improvements (II) Materials Management Gmp The european medicines agency's (ema) provides answers to frequently asked questions on good manufacturing practice (gmp) and good. Training in current good manufacturing practice shall be conducted by qualified individuals on a continuing basis and with sufficient frequency. In this guide “manufacturing” is defined to include all operations of receipt of materials, production, packaging, repackaging, labelling, relabelling,. Material portability, using. Materials Management Gmp.
From www.slideshare.net
Materials gmp Materials Management Gmp Good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of quality assurance. In this guide “manufacturing” is defined to include all operations of receipt of materials, production, packaging, repackaging, labelling, relabelling,. The european medicines agency's (ema) provides answers to frequently asked questions on good manufacturing practice (gmp) and good. Ich q11 defines. Materials Management Gmp.
From www.katsura-chemical.co.jp
Comply with GMP conditions for manufacturing safe and effective APIs Materials Management Gmp We focused on elements in ich q7 where further explanation and/or clarification are useful. Good manufacturing practice (gmp) is a system for ensuring that products are consistently produced and controlled according to quality standards. Ich q11 defines what to file as ‘api staring. In this guide “manufacturing” is defined to include all operations of receipt of materials, production, packaging, repackaging,. Materials Management Gmp.