Standard Heat Formation Temperature . 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Calculating heat of reaction from heat of formation. The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. An application of hess's law allows us to use standard heats of formation to indirectly. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard state heat of formation for the elemental form of each atom is zero. Standard enthalpies of formation (δh o f) are determined under standard conditions: A pressure of 1 atm for gases and a concentration of 1 m for species in solution, with all pure. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard.
from studylib.net
The standard state heat of formation for the elemental form of each atom is zero. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements. An application of hess's law allows us to use standard heats of formation to indirectly. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. A pressure of 1 atm for gases and a concentration of 1 m for species in solution, with all pure. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Calculating heat of reaction from heat of formation.
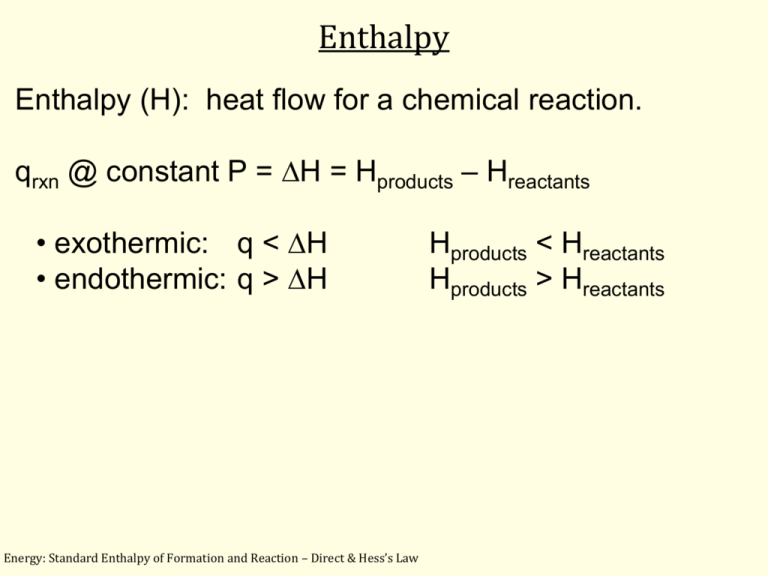
Standard Enthalpy of Formation and Reaction
Standard Heat Formation Temperature An application of hess's law allows us to use standard heats of formation to indirectly. The standard state heat of formation for the elemental form of each atom is zero. A pressure of 1 atm for gases and a concentration of 1 m for species in solution, with all pure. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements. Calculating heat of reaction from heat of formation. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. An application of hess's law allows us to use standard heats of formation to indirectly. Standard enthalpies of formation (δh o f) are determined under standard conditions:
From www.researchgate.net
Temperature variation with height according to the U.S. Standard Standard Heat Formation Temperature A pressure of 1 atm for gases and a concentration of 1 m for species in solution, with all pure. An application of hess's law allows us to use standard heats of formation to indirectly. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Standard. Standard Heat Formation Temperature.
From slideplayer.com
Transfers of energy as heat in chemical reactions and physical changes Standard Heat Formation Temperature The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Standard enthalpies of formation (δh o f) are determined under standard conditions: A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed. Standard Heat Formation Temperature.
From darkataxa.blogspot.com
Astounding Collections Of Heat Of Formation Table Photos Darkata Standard Heat Formation Temperature A pressure of 1 atm for gases and a concentration of 1 m for species in solution, with all pure. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1. Standard Heat Formation Temperature.
From www.slideserve.com
PPT Standard Heats of Reaction PowerPoint Presentation, free download Standard Heat Formation Temperature Standard enthalpies of formation (δh o f) are determined under standard conditions: 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. A. Standard Heat Formation Temperature.
From rayb78.github.io
Heat Of Formation Chart Standard Heat Formation Temperature A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. Calculating heat of reaction from heat of formation. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Standard enthalpies. Standard Heat Formation Temperature.
From www.youtube.com
Heat of Reaction (from Heat of Formation) YouTube Standard Heat Formation Temperature The standard state heat of formation for the elemental form of each atom is zero. Standard enthalpies of formation (δh o f) are determined under standard conditions: The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. A pressure of 1 atm for gases and a. Standard Heat Formation Temperature.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID314871 Standard Heat Formation Temperature 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation, also known as the heat of. Standard Heat Formation Temperature.
From www.ck12.org
Heating and Cooling Curves ( Read ) Chemistry CK12 Foundation Standard Heat Formation Temperature A pressure of 1 atm for gases and a concentration of 1 m for species in solution, with all pure. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation is a measure of the energy released or consumed. Standard Heat Formation Temperature.
From studylib.net
Standard Enthalpy of Formation and Reaction Standard Heat Formation Temperature Standard enthalpies of formation (δh o f) are determined under standard conditions: The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a. Standard Heat Formation Temperature.
From www.slideserve.com
PPT Advanced Thermodynamics Note 3 Heat Effects PowerPoint Standard Heat Formation Temperature Calculating heat of reaction from heat of formation. The standard state heat of formation for the elemental form of each atom is zero. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change. Standard Heat Formation Temperature.
From lessonluft.z19.web.core.windows.net
Heat Of Formation Chart Standard Heat Formation Temperature The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. An application of hess's law allows us to use standard heats of formation to indirectly. Calculating heat of reaction from heat of formation. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction. Standard Heat Formation Temperature.
From chemistry101efhs.weebly.com
Heat vs. Temperature Chemistry 101 Standard Heat Formation Temperature A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. An application of hess's law allows us to use standard heats of formation. Standard Heat Formation Temperature.
From www.researchgate.net
Enthalpy of formation and heats of formation of main combustion Standard Heat Formation Temperature 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard state heat of formation for the elemental form of each atom is zero. Standard enthalpies of formation (δh o f) are determined under standard conditions: The standard enthalpy of formation is a measure of the. Standard Heat Formation Temperature.
From rayb78.github.io
Heat Of Formation Chart Standard Heat Formation Temperature The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. An application of hess's law allows us to use standard heats of formation to indirectly. The standard enthalpy of formation is a measure of the energy released. Standard Heat Formation Temperature.
From brunofuga.adv.br
Standard Enthalpy Of Formation Definition, Table, Equation, 46 OFF Standard Heat Formation Temperature Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard state heat of formation for the elemental form of each atom is zero. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed. Standard Heat Formation Temperature.
From stahonorschemistry.weebly.com
III Calculating Enthalpies STA Form IV Honors Chemistry Standard Heat Formation Temperature 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. Calculating heat of reaction from heat of formation. The standard state. Standard Heat Formation Temperature.
From www.chegg.com
TABLE A286 Enthalpy of formation, Gibbs function of Standard Heat Formation Temperature The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements. An application of hess's law allows us to use standard heats of formation to indirectly. Calculating heat of reaction from heat of formation. The standard state heat of formation for the elemental form of each atom is zero. Standard enthalpies of. Standard Heat Formation Temperature.
From www.scribd.com
Standard Thermodynamic Values Standard Heat Formation Temperature An application of hess's law allows us to use standard heats of formation to indirectly. A pressure of 1 atm for gases and a concentration of 1 m for species in solution, with all pure. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from. Standard Heat Formation Temperature.
From www.chemistrylearner.com
Heat (Enthalpy) of Reaction Definition, Examples, & Formula Standard Heat Formation Temperature An application of hess's law allows us to use standard heats of formation to indirectly. The standard state heat of formation for the elemental form of each atom is zero. Standard enthalpies of formation (δh o f) are determined under standard conditions: A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole. Standard Heat Formation Temperature.
From lessonluft.z19.web.core.windows.net
Heat Of Formation Chart Standard Heat Formation Temperature Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. A pressure of 1 atm for gases and a concentration of 1 m for species in solution, with all pure. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole. Standard Heat Formation Temperature.
From www.geeksforgeeks.org
Difference Between Heat and Temperature SI Unit, Measurement, FAQs Standard Heat Formation Temperature The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. A pressure of 1 atm for gases and a concentration of 1 m for species in solution, with all pure.. Standard Heat Formation Temperature.
From slideplayer.com
Chapter 10 “Thermochemistry” ppt download Standard Heat Formation Temperature 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction. Standard Heat Formation Temperature.
From mungfali.com
Standard Heat Formation Chart Standard Heat Formation Temperature The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when. Standard Heat Formation Temperature.
From www.slideserve.com
PPT Thermal Energy PowerPoint Presentation, free download ID5769943 Standard Heat Formation Temperature The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which. Standard Heat Formation Temperature.
From www.youtube.com
Std Heat of Formation versus Bond Energy YouTube Standard Heat Formation Temperature An application of hess's law allows us to use standard heats of formation to indirectly. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard state heat of formation for the elemental form of each atom is zero. Standard enthalpies of formation (δh o. Standard Heat Formation Temperature.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Standard Heat Formation Temperature Standard enthalpies of formation (δh o f) are determined under standard conditions: An application of hess's law allows us to use standard heats of formation to indirectly. The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements. Standard enthalpy change of formation (data table) these tables include heat of formation data. Standard Heat Formation Temperature.
From www.slideserve.com
PPT STANDARD HEAT OF FORMATION ΔH 0 f or ΔH θ f PowerPoint Standard Heat Formation Temperature Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a. Standard Heat Formation Temperature.
From www.semanticscholar.org
Table 3 from Standard thermodynamic properties of H3PO4(aq) over a wide Standard Heat Formation Temperature Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Calculating heat of reaction from heat of formation. The standard state heat of formation for the. Standard Heat Formation Temperature.
From www.showme.com
Standard heat of formation Science, Chemistry, thermochemistry ShowMe Standard Heat Formation Temperature A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard state heat of formation for the elemental form of each atom is zero. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole. Standard Heat Formation Temperature.
From www.researchgate.net
Standard free energy of formation of metal oxides as a function of Standard Heat Formation Temperature An application of hess's law allows us to use standard heats of formation to indirectly. Standard enthalpies of formation (δh o f) are determined under standard conditions: The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure.. Standard Heat Formation Temperature.
From www.nagwa.com
Question Video Calculating the Standard Heat of Reaction for the Standard Heat Formation Temperature The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements. Standard enthalpies of formation (δh o f) are determined under standard conditions: A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard state. Standard Heat Formation Temperature.
From lessonsybil.z5.web.core.windows.net
How To Calculate The Heat Of Formation Standard Heat Formation Temperature The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Calculating heat of reaction from heat of formation. The standard enthalpy of formation, also known. Standard Heat Formation Temperature.
From abc30.com
What is a heat wave? How heat waves form and temperatures climb ABC30 Standard Heat Formation Temperature The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements. A pressure of 1 atm for gases and a concentration of 1 m for species in solution, with all pure. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure. Standard Heat Formation Temperature.
From www.youtube.com
Standard heat of formation problem / Heat of formation formation Standard Heat Formation Temperature Calculating heat of reaction from heat of formation. The standard state heat of formation for the elemental form of each atom is zero. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of. Standard Heat Formation Temperature.
From lessonlibsertularia.z22.web.core.windows.net
How To Determine The Heat Of Formation Standard Heat Formation Temperature The standard state heat of formation for the elemental form of each atom is zero. An application of hess's law allows us to use standard heats of formation to indirectly. Standard enthalpies of formation (δh o f) are determined under standard conditions: Calculating heat of reaction from heat of formation. A standard enthalpy of formation $δh°_f$ is an enthalpy change. Standard Heat Formation Temperature.