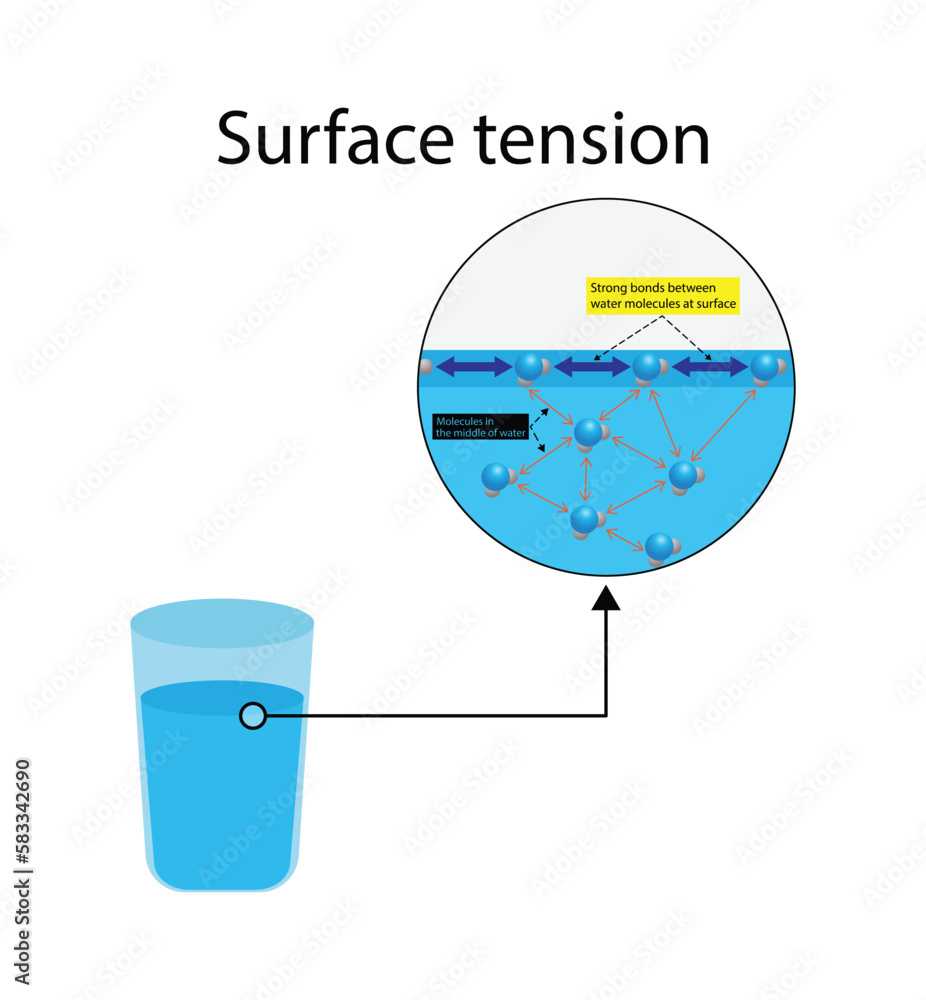
What Causes Surface Tension In Water . Learn how water molecules attract each other and form a strong bond at the surface, creating surface tension. See a video of an. Learn how water molecules attract each other and other substances, and how this affects phenomena such as capillary action, meniscus, and water striders. Surface tension is the result of intermolecular forces that hold the liquid molecules together. Surface tension is the property of a liquid surface that acts like a stretched elastic membrane. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.” surface tension forces are caused by intermolecular forces between the molecules of a liquid at its outer boundaries. Water's surface tension is predominantly a consequence of the potent hydrogen bonds formed between its molecules, while mercury's surface tension is attributable to the substantially stronger metallic bonds exhibited by its molecular structure. It depends on the forces of attraction between the molecules and the gas, solid,. Surface tension is the energy required to increase the surface area of a liquid due to intermolecular forces. See examples of surface tension in nature, science, and everyday. Learn how polarity, hydrogen bonding, cohesive and adhesive forces affect surface. Learn how the hydrogen bond causes water to have high surface tension, which affects its properties and behaviors.
from stock.adobe.com
Surface tension is the property of a liquid surface that acts like a stretched elastic membrane. Water's surface tension is predominantly a consequence of the potent hydrogen bonds formed between its molecules, while mercury's surface tension is attributable to the substantially stronger metallic bonds exhibited by its molecular structure. See examples of surface tension in nature, science, and everyday. Surface tension is the result of intermolecular forces that hold the liquid molecules together. Surface tension is the energy required to increase the surface area of a liquid due to intermolecular forces. See a video of an. Learn how the hydrogen bond causes water to have high surface tension, which affects its properties and behaviors. It depends on the forces of attraction between the molecules and the gas, solid,. Learn how water molecules attract each other and other substances, and how this affects phenomena such as capillary action, meniscus, and water striders. Learn how polarity, hydrogen bonding, cohesive and adhesive forces affect surface.
illustration of physics, Surface tension of water, the cohesive forces
What Causes Surface Tension In Water Surface tension is the property of a liquid surface that acts like a stretched elastic membrane. It depends on the forces of attraction between the molecules and the gas, solid,. Water's surface tension is predominantly a consequence of the potent hydrogen bonds formed between its molecules, while mercury's surface tension is attributable to the substantially stronger metallic bonds exhibited by its molecular structure. See a video of an. Learn how water molecules attract each other and other substances, and how this affects phenomena such as capillary action, meniscus, and water striders. See examples of surface tension in nature, science, and everyday. Surface tension is the energy required to increase the surface area of a liquid due to intermolecular forces. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.” surface tension forces are caused by intermolecular forces between the molecules of a liquid at its outer boundaries. Learn how water molecules attract each other and form a strong bond at the surface, creating surface tension. Surface tension is the property of a liquid surface that acts like a stretched elastic membrane. Learn how polarity, hydrogen bonding, cohesive and adhesive forces affect surface. Learn how the hydrogen bond causes water to have high surface tension, which affects its properties and behaviors. Surface tension is the result of intermolecular forces that hold the liquid molecules together.
From stock.adobe.com
illustration of physics, Surface tension of water, the cohesive forces What Causes Surface Tension In Water Learn how water molecules attract each other and form a strong bond at the surface, creating surface tension. Learn how polarity, hydrogen bonding, cohesive and adhesive forces affect surface. See examples of surface tension in nature, science, and everyday. Surface tension is the property of a liquid surface that acts like a stretched elastic membrane. Surface tension is the result. What Causes Surface Tension In Water.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts What Causes Surface Tension In Water “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.” surface tension forces are caused by intermolecular forces between the molecules of a liquid at its outer boundaries. Learn how the hydrogen bond causes water to have high surface. What Causes Surface Tension In Water.
From ar.inspiredpencil.com
What Causes Surface Tension In Water What Causes Surface Tension In Water Surface tension is the energy required to increase the surface area of a liquid due to intermolecular forces. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.” surface tension forces are caused by intermolecular forces between the molecules. What Causes Surface Tension In Water.
From upberi.com
Surface Tension Definition, Formula, Causes, Examples, and FAQs (2023) What Causes Surface Tension In Water See examples of surface tension in nature, science, and everyday. See a video of an. It depends on the forces of attraction between the molecules and the gas, solid,. Learn how water molecules attract each other and form a strong bond at the surface, creating surface tension. Water's surface tension is predominantly a consequence of the potent hydrogen bonds formed. What Causes Surface Tension In Water.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance What Causes Surface Tension In Water Learn how the hydrogen bond causes water to have high surface tension, which affects its properties and behaviors. See examples of surface tension in nature, science, and everyday. Learn how water molecules attract each other and other substances, and how this affects phenomena such as capillary action, meniscus, and water striders. Learn how water molecules attract each other and form. What Causes Surface Tension In Water.
From www.youtube.com
Surface Tension What is it, how does it form, what properties does it What Causes Surface Tension In Water Surface tension is the property of a liquid surface that acts like a stretched elastic membrane. Surface tension is the result of intermolecular forces that hold the liquid molecules together. Water's surface tension is predominantly a consequence of the potent hydrogen bonds formed between its molecules, while mercury's surface tension is attributable to the substantially stronger metallic bonds exhibited by. What Causes Surface Tension In Water.
From www.youtube.com
Surface Tension of Water Explained YouTube What Causes Surface Tension In Water It depends on the forces of attraction between the molecules and the gas, solid,. Surface tension is the result of intermolecular forces that hold the liquid molecules together. Learn how water molecules attract each other and other substances, and how this affects phenomena such as capillary action, meniscus, and water striders. Learn how polarity, hydrogen bonding, cohesive and adhesive forces. What Causes Surface Tension In Water.
From www.slideserve.com
PPT Chapter 15 PowerPoint Presentation ID245553 What Causes Surface Tension In Water Learn how water molecules attract each other and other substances, and how this affects phenomena such as capillary action, meniscus, and water striders. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.” surface tension forces are caused by. What Causes Surface Tension In Water.
From techblog.ctgclean.com
What is Surface Tension? CTG Technical Blog What Causes Surface Tension In Water See a video of an. Learn how water molecules attract each other and form a strong bond at the surface, creating surface tension. Learn how water molecules attract each other and other substances, and how this affects phenomena such as capillary action, meniscus, and water striders. Surface tension is the property of a liquid surface that acts like a stretched. What Causes Surface Tension In Water.
From mavink.com
Water Molecules Surface Tension What Causes Surface Tension In Water Learn how water molecules attract each other and other substances, and how this affects phenomena such as capillary action, meniscus, and water striders. It depends on the forces of attraction between the molecules and the gas, solid,. See a video of an. Water's surface tension is predominantly a consequence of the potent hydrogen bonds formed between its molecules, while mercury's. What Causes Surface Tension In Water.
From factsandhistory.com
Why Does Water Feel Like Concrete When You Belly Flop Into It What Causes Surface Tension In Water “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.” surface tension forces are caused by intermolecular forces between the molecules of a liquid at its outer boundaries. Learn how polarity, hydrogen bonding, cohesive and adhesive forces affect surface.. What Causes Surface Tension In Water.
From www.slideshare.net
Measuring the Surface Tension of Water by Light Diffraction on Capill… What Causes Surface Tension In Water Surface tension is the energy required to increase the surface area of a liquid due to intermolecular forces. Surface tension is the property of a liquid surface that acts like a stretched elastic membrane. Water's surface tension is predominantly a consequence of the potent hydrogen bonds formed between its molecules, while mercury's surface tension is attributable to the substantially stronger. What Causes Surface Tension In Water.
From www.thoughtco.com
What Is Surface Tension? Definition and Experiments What Causes Surface Tension In Water Learn how water molecules attract each other and other substances, and how this affects phenomena such as capillary action, meniscus, and water striders. Learn how the hydrogen bond causes water to have high surface tension, which affects its properties and behaviors. Surface tension is the property of a liquid surface that acts like a stretched elastic membrane. Surface tension is. What Causes Surface Tension In Water.
From ar.inspiredpencil.com
What Causes Surface Tension In Water What Causes Surface Tension In Water Surface tension is the energy required to increase the surface area of a liquid due to intermolecular forces. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.” surface tension forces are caused by intermolecular forces between the molecules. What Causes Surface Tension In Water.
From www.slideserve.com
PPT Water and Aqueous Systems PowerPoint Presentation ID565121 What Causes Surface Tension In Water Learn how the hydrogen bond causes water to have high surface tension, which affects its properties and behaviors. Learn how water molecules attract each other and form a strong bond at the surface, creating surface tension. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface. What Causes Surface Tension In Water.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance What Causes Surface Tension In Water “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.” surface tension forces are caused by intermolecular forces between the molecules of a liquid at its outer boundaries. See examples of surface tension in nature, science, and everyday. Learn. What Causes Surface Tension In Water.
From ar.inspiredpencil.com
What Causes Surface Tension In Water What Causes Surface Tension In Water Water's surface tension is predominantly a consequence of the potent hydrogen bonds formed between its molecules, while mercury's surface tension is attributable to the substantially stronger metallic bonds exhibited by its molecular structure. It depends on the forces of attraction between the molecules and the gas, solid,. Surface tension is the result of intermolecular forces that hold the liquid molecules. What Causes Surface Tension In Water.
From study.com
Surface Tension Definition, Calculation & Examples Video & Lesson What Causes Surface Tension In Water It depends on the forces of attraction between the molecules and the gas, solid,. See examples of surface tension in nature, science, and everyday. Learn how water molecules attract each other and form a strong bond at the surface, creating surface tension. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction. What Causes Surface Tension In Water.
From www.slideserve.com
PPT Water PowerPoint Presentation, free download ID2600281 What Causes Surface Tension In Water It depends on the forces of attraction between the molecules and the gas, solid,. Learn how water molecules attract each other and form a strong bond at the surface, creating surface tension. Water's surface tension is predominantly a consequence of the potent hydrogen bonds formed between its molecules, while mercury's surface tension is attributable to the substantially stronger metallic bonds. What Causes Surface Tension In Water.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs What Causes Surface Tension In Water See a video of an. Learn how polarity, hydrogen bonding, cohesive and adhesive forces affect surface. It depends on the forces of attraction between the molecules and the gas, solid,. Water's surface tension is predominantly a consequence of the potent hydrogen bonds formed between its molecules, while mercury's surface tension is attributable to the substantially stronger metallic bonds exhibited by. What Causes Surface Tension In Water.
From funsizephysics.com
What is Surface Tension? FunsizePhysics What Causes Surface Tension In Water See a video of an. Surface tension is the property of a liquid surface that acts like a stretched elastic membrane. Learn how water molecules attract each other and form a strong bond at the surface, creating surface tension. Learn how polarity, hydrogen bonding, cohesive and adhesive forces affect surface. It depends on the forces of attraction between the molecules. What Causes Surface Tension In Water.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance What Causes Surface Tension In Water Surface tension is the result of intermolecular forces that hold the liquid molecules together. Learn how water molecules attract each other and other substances, and how this affects phenomena such as capillary action, meniscus, and water striders. Surface tension is the energy required to increase the surface area of a liquid due to intermolecular forces. See examples of surface tension. What Causes Surface Tension In Water.
From www.biolinscientific.com
Surface tension of water Why is it so high? What Causes Surface Tension In Water Learn how the hydrogen bond causes water to have high surface tension, which affects its properties and behaviors. Water's surface tension is predominantly a consequence of the potent hydrogen bonds formed between its molecules, while mercury's surface tension is attributable to the substantially stronger metallic bonds exhibited by its molecular structure. Surface tension is the property of a liquid surface. What Causes Surface Tension In Water.
From www.dreamstime.com
Surface Tension Explanation Vector Illustration Diagram Stock Vector What Causes Surface Tension In Water Learn how water molecules attract each other and form a strong bond at the surface, creating surface tension. See examples of surface tension in nature, science, and everyday. Surface tension is the property of a liquid surface that acts like a stretched elastic membrane. Learn how water molecules attract each other and other substances, and how this affects phenomena such. What Causes Surface Tension In Water.
From www.elephango.com
Tension in the Water Educational Resources K12 Learning, Physical What Causes Surface Tension In Water Surface tension is the result of intermolecular forces that hold the liquid molecules together. Learn how the hydrogen bond causes water to have high surface tension, which affects its properties and behaviors. Surface tension is the energy required to increase the surface area of a liquid due to intermolecular forces. Learn how polarity, hydrogen bonding, cohesive and adhesive forces affect. What Causes Surface Tension In Water.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs What Causes Surface Tension In Water Surface tension is the result of intermolecular forces that hold the liquid molecules together. See examples of surface tension in nature, science, and everyday. Learn how water molecules attract each other and other substances, and how this affects phenomena such as capillary action, meniscus, and water striders. Surface tension is the energy required to increase the surface area of a. What Causes Surface Tension In Water.
From www.youtube.com
What is surface tension of water explained in detail? YouTube What Causes Surface Tension In Water Learn how water molecules attract each other and other substances, and how this affects phenomena such as capillary action, meniscus, and water striders. Surface tension is the energy required to increase the surface area of a liquid due to intermolecular forces. Surface tension is the property of a liquid surface that acts like a stretched elastic membrane. See examples of. What Causes Surface Tension In Water.
From www.slideserve.com
PPT Liquids PowerPoint Presentation, free download ID9168408 What Causes Surface Tension In Water Learn how water molecules attract each other and other substances, and how this affects phenomena such as capillary action, meniscus, and water striders. Surface tension is the energy required to increase the surface area of a liquid due to intermolecular forces. See examples of surface tension in nature, science, and everyday. It depends on the forces of attraction between the. What Causes Surface Tension In Water.
From www.slideserve.com
PPT Surface Tension PowerPoint Presentation, free download ID3106425 What Causes Surface Tension In Water Surface tension is the result of intermolecular forces that hold the liquid molecules together. Surface tension is the energy required to increase the surface area of a liquid due to intermolecular forces. Water's surface tension is predominantly a consequence of the potent hydrogen bonds formed between its molecules, while mercury's surface tension is attributable to the substantially stronger metallic bonds. What Causes Surface Tension In Water.
From www.expii.com
Surface Tension of Water — Overview & Importance Expii What Causes Surface Tension In Water It depends on the forces of attraction between the molecules and the gas, solid,. Learn how polarity, hydrogen bonding, cohesive and adhesive forces affect surface. Surface tension is the energy required to increase the surface area of a liquid due to intermolecular forces. Water's surface tension is predominantly a consequence of the potent hydrogen bonds formed between its molecules, while. What Causes Surface Tension In Water.
From byjus.com
Explain the surface tension phenomenon with examples. What Causes Surface Tension In Water “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.” surface tension forces are caused by intermolecular forces between the molecules of a liquid at its outer boundaries. Surface tension is the result of intermolecular forces that hold the. What Causes Surface Tension In Water.
From ar.inspiredpencil.com
Surface Tension Of Water Diagram What Causes Surface Tension In Water Surface tension is the energy required to increase the surface area of a liquid due to intermolecular forces. See examples of surface tension in nature, science, and everyday. Water's surface tension is predominantly a consequence of the potent hydrogen bonds formed between its molecules, while mercury's surface tension is attributable to the substantially stronger metallic bonds exhibited by its molecular. What Causes Surface Tension In Water.
From blog.merocourse.com
What is Surface Tension? Factors Affecting Surface Tension Merocourse What Causes Surface Tension In Water Surface tension is the property of a liquid surface that acts like a stretched elastic membrane. See a video of an. Surface tension is the result of intermolecular forces that hold the liquid molecules together. Learn how water molecules attract each other and other substances, and how this affects phenomena such as capillary action, meniscus, and water striders. It depends. What Causes Surface Tension In Water.
From www.youtube.com
DETERMINING THE SURFACE TENSION OF WATER EASY TO UNDERSTAND YouTube What Causes Surface Tension In Water Water's surface tension is predominantly a consequence of the potent hydrogen bonds formed between its molecules, while mercury's surface tension is attributable to the substantially stronger metallic bonds exhibited by its molecular structure. See examples of surface tension in nature, science, and everyday. Surface tension is the result of intermolecular forces that hold the liquid molecules together. Learn how water. What Causes Surface Tension In Water.
From www.slideserve.com
PPT Chapter 15 PowerPoint Presentation ID245553 What Causes Surface Tension In Water See examples of surface tension in nature, science, and everyday. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.” surface tension forces are caused by intermolecular forces between the molecules of a liquid at its outer boundaries. See. What Causes Surface Tension In Water.