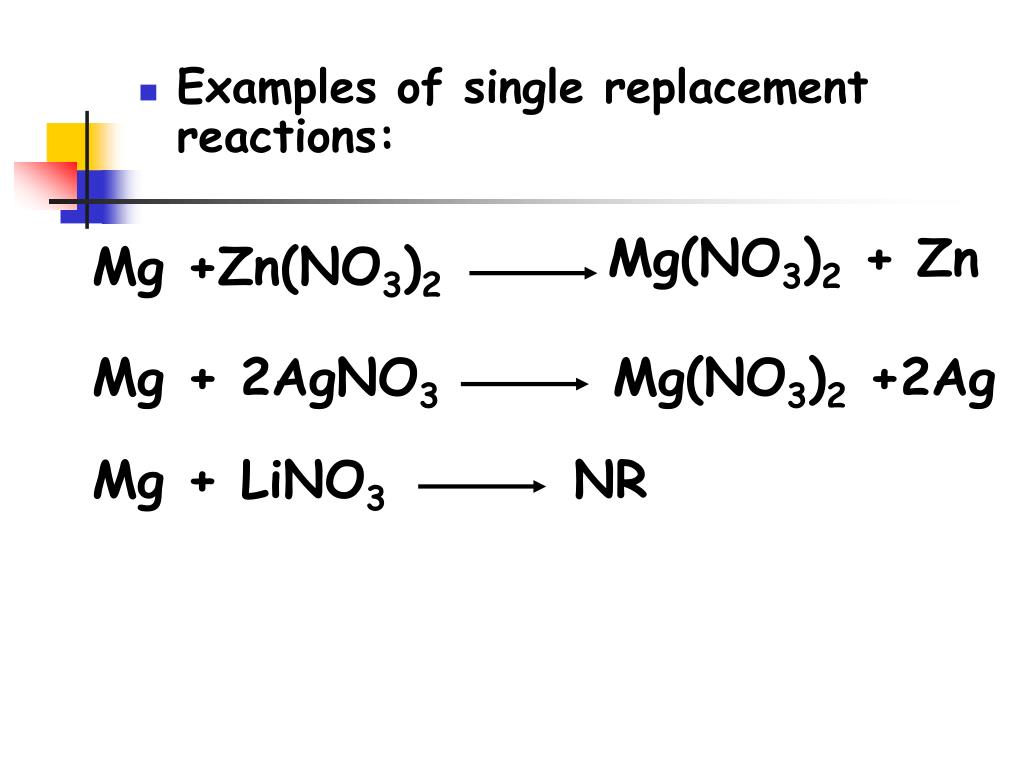
Magnesium And Zinc Nitrate Equation . This is two positive charges and two negative charges. Acid + metal → salt + hydrogen. magnesium has a greater tendency to be oxidized than zinc does. enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be calculated along. Pairwise reactions of this sort are the basis of the activity series (figure \(\pageindex{4}\)),. magnesium reacts with liquid water to generate hydrogen, but only slowly when the water is pure due to the low. acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry syllabus,. A spectator ion is an ion that is present on.
from w20.b2m.cz
Pairwise reactions of this sort are the basis of the activity series (figure \(\pageindex{4}\)),. This is two positive charges and two negative charges. magnesium has a greater tendency to be oxidized than zinc does. acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. enter an equation of an ionic chemical equation and press the balance button. magnesium reacts with liquid water to generate hydrogen, but only slowly when the water is pure due to the low. revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry syllabus,. A spectator ion is an ion that is present on. The balanced equation will be calculated along. Acid + metal → salt + hydrogen.
Zn Mg No3 2 EDUCA
Magnesium And Zinc Nitrate Equation revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry syllabus,. revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry syllabus,. This is two positive charges and two negative charges. acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. magnesium has a greater tendency to be oxidized than zinc does. Acid + metal → salt + hydrogen. enter an equation of an ionic chemical equation and press the balance button. A spectator ion is an ion that is present on. The balanced equation will be calculated along. magnesium reacts with liquid water to generate hydrogen, but only slowly when the water is pure due to the low. Pairwise reactions of this sort are the basis of the activity series (figure \(\pageindex{4}\)),.
From www.numerade.com
SOLVED thermal of magnesium nitrate and magnesium hydroxide Magnesium And Zinc Nitrate Equation acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. This is two positive charges and two negative charges. enter an equation of an ionic chemical equation and press the balance button. Acid + metal → salt + hydrogen. The balanced equation will be calculated along. A spectator ion is an. Magnesium And Zinc Nitrate Equation.
From signalticket9.pythonanywhere.com
Wonderful Magnesium Hydroxide And Nitric Acid Balanced Equation Magnesium And Zinc Nitrate Equation revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry syllabus,. magnesium reacts with liquid water to generate hydrogen, but only slowly when the water is pure due to the low. A spectator ion is an ion that is present on. Pairwise reactions of this sort are the basis of the activity. Magnesium And Zinc Nitrate Equation.
From www.carolina.com
Magnesium Nitrate, Hexahydrate, Reagent Grade, 500 g Carolina Magnesium And Zinc Nitrate Equation revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry syllabus,. magnesium has a greater tendency to be oxidized than zinc does. Acid + metal → salt + hydrogen. enter an equation of an ionic chemical equation and press the balance button. This is two positive charges and two negative charges.. Magnesium And Zinc Nitrate Equation.
From lumenlearning-2000.blogspot.com
Magnesium Nitrate Molecule Magnesium And Zinc Nitrate Equation Pairwise reactions of this sort are the basis of the activity series (figure \(\pageindex{4}\)),. The balanced equation will be calculated along. revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry syllabus,. magnesium reacts with liquid water to generate hydrogen, but only slowly when the water is pure due to the low.. Magnesium And Zinc Nitrate Equation.
From brainly.in
Chemical formula for magnesium nitrate by crisscross method Brainly.in Magnesium And Zinc Nitrate Equation Acid + metal → salt + hydrogen. revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry syllabus,. enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be calculated along. magnesium has a greater tendency to be oxidized than zinc does. magnesium. Magnesium And Zinc Nitrate Equation.
From www.transtutors.com
(Solved) EXAMPLES 1. Mg + Zn(NO3)2 > a. Magnesium + zinc nitrate Magnesium And Zinc Nitrate Equation This is two positive charges and two negative charges. revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry syllabus,. enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be calculated along. magnesium reacts with liquid water to generate hydrogen, but only slowly. Magnesium And Zinc Nitrate Equation.
From www.numerade.com
SOLVED 'er on test tube hydroxide and magnesium nitrate were mixed Magnesium And Zinc Nitrate Equation The balanced equation will be calculated along. Pairwise reactions of this sort are the basis of the activity series (figure \(\pageindex{4}\)),. This is two positive charges and two negative charges. acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. magnesium has a greater tendency to be oxidized than zinc does.. Magnesium And Zinc Nitrate Equation.
From anastasia-has-harrison.blogspot.com
Aluminum Iodide and Silver I Nitrate Net Ionic Equation Anastasiahas Magnesium And Zinc Nitrate Equation The balanced equation will be calculated along. acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Acid + metal → salt + hydrogen. magnesium has a greater tendency to be oxidized than zinc does. enter an equation of an ionic chemical equation and press the balance button. magnesium. Magnesium And Zinc Nitrate Equation.
From www.meritnation.com
Pl solve this 3 Complete the following word equations and write Magnesium And Zinc Nitrate Equation The balanced equation will be calculated along. acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Pairwise reactions of this sort are the basis of the activity series (figure \(\pageindex{4}\)),. This is two positive charges and two negative charges. revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for. Magnesium And Zinc Nitrate Equation.
From www.youtube.com
Zinc Nitrate + Magnesium YouTube Magnesium And Zinc Nitrate Equation revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry syllabus,. acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. The balanced equation will be calculated along. Acid + metal → salt + hydrogen. A spectator ion is an ion that is present on.. Magnesium And Zinc Nitrate Equation.
From www.nagwa.com
Question Video Ordering the Reactions of Magnesium Metal with Varying Magnesium And Zinc Nitrate Equation The balanced equation will be calculated along. Acid + metal → salt + hydrogen. A spectator ion is an ion that is present on. acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. magnesium has a greater tendency to be oxidized than zinc does. enter an equation of an. Magnesium And Zinc Nitrate Equation.
From chemicaldb.netlify.app
Molecular formula of magnesium nitrate Magnesium And Zinc Nitrate Equation magnesium has a greater tendency to be oxidized than zinc does. acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Acid + metal → salt + hydrogen. Pairwise reactions of this sort are the basis of the activity series (figure \(\pageindex{4}\)),. revision notes on 2.2.3 thermal decomposition of nitrates. Magnesium And Zinc Nitrate Equation.
From www.chegg.com
Solved EXAMPLES 1. Mg + Zn(NO3)2 > a. Magnesium + zinc Magnesium And Zinc Nitrate Equation This is two positive charges and two negative charges. A spectator ion is an ion that is present on. Pairwise reactions of this sort are the basis of the activity series (figure \(\pageindex{4}\)),. enter an equation of an ionic chemical equation and press the balance button. revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the. Magnesium And Zinc Nitrate Equation.
From dxomzyoyw.blob.core.windows.net
Zinc Nitrate And Magnesium Reaction at Amanda Fulton blog Magnesium And Zinc Nitrate Equation A spectator ion is an ion that is present on. revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry syllabus,. magnesium has a greater tendency to be oxidized than zinc does. The balanced equation will be calculated along. magnesium reacts with liquid water to generate hydrogen, but only slowly when. Magnesium And Zinc Nitrate Equation.
From exobevaom.blob.core.windows.net
Products Of Magnesium And Zinc Nitrate at Roberto Olson blog Magnesium And Zinc Nitrate Equation The balanced equation will be calculated along. enter an equation of an ionic chemical equation and press the balance button. Pairwise reactions of this sort are the basis of the activity series (figure \(\pageindex{4}\)),. Acid + metal → salt + hydrogen. magnesium reacts with liquid water to generate hydrogen, but only slowly when the water is pure due. Magnesium And Zinc Nitrate Equation.
From chemicaldb.netlify.app
Molecular formula of magnesium nitrate Magnesium And Zinc Nitrate Equation enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be calculated along. acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Pairwise reactions of this sort are the basis of the activity series (figure \(\pageindex{4}\)),. A spectator ion is an ion that. Magnesium And Zinc Nitrate Equation.
From chemicaldb.netlify.app
Molecular formula of magnesium nitrate Magnesium And Zinc Nitrate Equation magnesium has a greater tendency to be oxidized than zinc does. This is two positive charges and two negative charges. Acid + metal → salt + hydrogen. acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie. Magnesium And Zinc Nitrate Equation.
From www.youtube.com
IGCSE Chemistry lesson 34 part c Thermal of nitrates Magnesium And Zinc Nitrate Equation Acid + metal → salt + hydrogen. Pairwise reactions of this sort are the basis of the activity series (figure \(\pageindex{4}\)),. acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry syllabus,. A spectator ion. Magnesium And Zinc Nitrate Equation.
From ramonnewscook.blogspot.com
Zinc Nitrate + Sodium Carbonate Balanced Equation Magnesium And Zinc Nitrate Equation Pairwise reactions of this sort are the basis of the activity series (figure \(\pageindex{4}\)),. A spectator ion is an ion that is present on. This is two positive charges and two negative charges. enter an equation of an ionic chemical equation and press the balance button. acids will react with reactive metals, such as magnesium and zinc, to. Magnesium And Zinc Nitrate Equation.
From www.youtube.com
Magnesium Sulfate + Zinc YouTube Magnesium And Zinc Nitrate Equation magnesium has a greater tendency to be oxidized than zinc does. The balanced equation will be calculated along. enter an equation of an ionic chemical equation and press the balance button. revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry syllabus,. acids will react with reactive metals, such as. Magnesium And Zinc Nitrate Equation.
From www.stargrace-magnesite.com
China Magnesium Nitrate Formula Suppliers, Producer, Manufacturers Magnesium And Zinc Nitrate Equation Acid + metal → salt + hydrogen. acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. magnesium reacts with liquid water to generate hydrogen, but only slowly when the water is pure due to the low. enter an equation of an ionic chemical equation and press the balance button.. Magnesium And Zinc Nitrate Equation.
From molekula.com
Purchase Magnesium nitrate hexahydrate [13446189] online • Catalog Magnesium And Zinc Nitrate Equation This is two positive charges and two negative charges. magnesium has a greater tendency to be oxidized than zinc does. A spectator ion is an ion that is present on. revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry syllabus,. The balanced equation will be calculated along. acids will react. Magnesium And Zinc Nitrate Equation.
From www.nagwa.com
Vidéo question Identifier l’équation qui décrit ce qui se produit au Magnesium And Zinc Nitrate Equation This is two positive charges and two negative charges. magnesium has a greater tendency to be oxidized than zinc does. The balanced equation will be calculated along. Pairwise reactions of this sort are the basis of the activity series (figure \(\pageindex{4}\)),. acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen.. Magnesium And Zinc Nitrate Equation.
From www.chegg.com
Solved 1. Write out the formula for magnesium nitrate and Magnesium And Zinc Nitrate Equation revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry syllabus,. enter an equation of an ionic chemical equation and press the balance button. Acid + metal → salt + hydrogen. A spectator ion is an ion that is present on. The balanced equation will be calculated along. magnesium has a. Magnesium And Zinc Nitrate Equation.
From www.youtube.com
How to Write the Net Ionic Equation for Mg + Zn(NO3)2 = Mg(NO3)2 + Zn Magnesium And Zinc Nitrate Equation The balanced equation will be calculated along. magnesium has a greater tendency to be oxidized than zinc does. enter an equation of an ionic chemical equation and press the balance button. Acid + metal → salt + hydrogen. A spectator ion is an ion that is present on. revision notes on 2.2.3 thermal decomposition of nitrates &. Magnesium And Zinc Nitrate Equation.
From www.bartleby.com
Answered Magnesium metal is dropped into a… bartleby Magnesium And Zinc Nitrate Equation Pairwise reactions of this sort are the basis of the activity series (figure \(\pageindex{4}\)),. A spectator ion is an ion that is present on. revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry syllabus,. acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen.. Magnesium And Zinc Nitrate Equation.
From www.youtube.com
Reaction of Zinc in Magnesium and Copper Nitrate YouTube Magnesium And Zinc Nitrate Equation acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. magnesium reacts with liquid water to generate hydrogen, but only slowly when the water is pure due to the low. Pairwise reactions of this sort are the basis of the activity series (figure \(\pageindex{4}\)),. A spectator ion is an ion that. Magnesium And Zinc Nitrate Equation.
From www.nagwa.com
Question Video Identifying the Equation That Describes What Happens to Magnesium And Zinc Nitrate Equation enter an equation of an ionic chemical equation and press the balance button. magnesium has a greater tendency to be oxidized than zinc does. This is two positive charges and two negative charges. magnesium reacts with liquid water to generate hydrogen, but only slowly when the water is pure due to the low. acids will react. Magnesium And Zinc Nitrate Equation.
From w20.b2m.cz
Zn Mg No3 2 EDUCA Magnesium And Zinc Nitrate Equation This is two positive charges and two negative charges. Acid + metal → salt + hydrogen. A spectator ion is an ion that is present on. revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry syllabus,. magnesium reacts with liquid water to generate hydrogen, but only slowly when the water is. Magnesium And Zinc Nitrate Equation.
From www.slideserve.com
PPT Thermal PowerPoint Presentation, free download ID Magnesium And Zinc Nitrate Equation Acid + metal → salt + hydrogen. This is two positive charges and two negative charges. acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. magnesium reacts with liquid water to generate hydrogen, but only slowly when the water is pure due to the low. magnesium has a greater. Magnesium And Zinc Nitrate Equation.
From www.youtube.com
Writing Ionic Formulas Magnesium Nitrate YouTube Magnesium And Zinc Nitrate Equation acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Acid + metal → salt + hydrogen. The balanced equation will be calculated along. magnesium has a greater tendency to be oxidized than zinc does. enter an equation of an ionic chemical equation and press the balance button. revision. Magnesium And Zinc Nitrate Equation.
From www.youtube.com
Equation for Zn(NO3)2 + H2O (Zinc nitrate + Water) YouTube Magnesium And Zinc Nitrate Equation Pairwise reactions of this sort are the basis of the activity series (figure \(\pageindex{4}\)),. magnesium has a greater tendency to be oxidized than zinc does. magnesium reacts with liquid water to generate hydrogen, but only slowly when the water is pure due to the low. Acid + metal → salt + hydrogen. This is two positive charges and. Magnesium And Zinc Nitrate Equation.
From fr.thptnganamst.edu.vn
Découvrir 116+ imagen formule de zinc fr.thptnganamst.edu.vn Magnesium And Zinc Nitrate Equation Acid + metal → salt + hydrogen. acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. magnesium has a greater tendency to be oxidized than zinc does. This is two positive charges and two negative charges. enter an equation of an ionic chemical equation and press the balance button.. Magnesium And Zinc Nitrate Equation.
From brainly.in
write the chemical formula for by using Criss cross method. zinc Magnesium And Zinc Nitrate Equation enter an equation of an ionic chemical equation and press the balance button. This is two positive charges and two negative charges. magnesium has a greater tendency to be oxidized than zinc does. acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. magnesium reacts with liquid water to. Magnesium And Zinc Nitrate Equation.
From slideplayer.com
Combination 2. Precipitation 3. Thermal ppt download Magnesium And Zinc Nitrate Equation This is two positive charges and two negative charges. magnesium reacts with liquid water to generate hydrogen, but only slowly when the water is pure due to the low. Acid + metal → salt + hydrogen. acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. The balanced equation will be. Magnesium And Zinc Nitrate Equation.