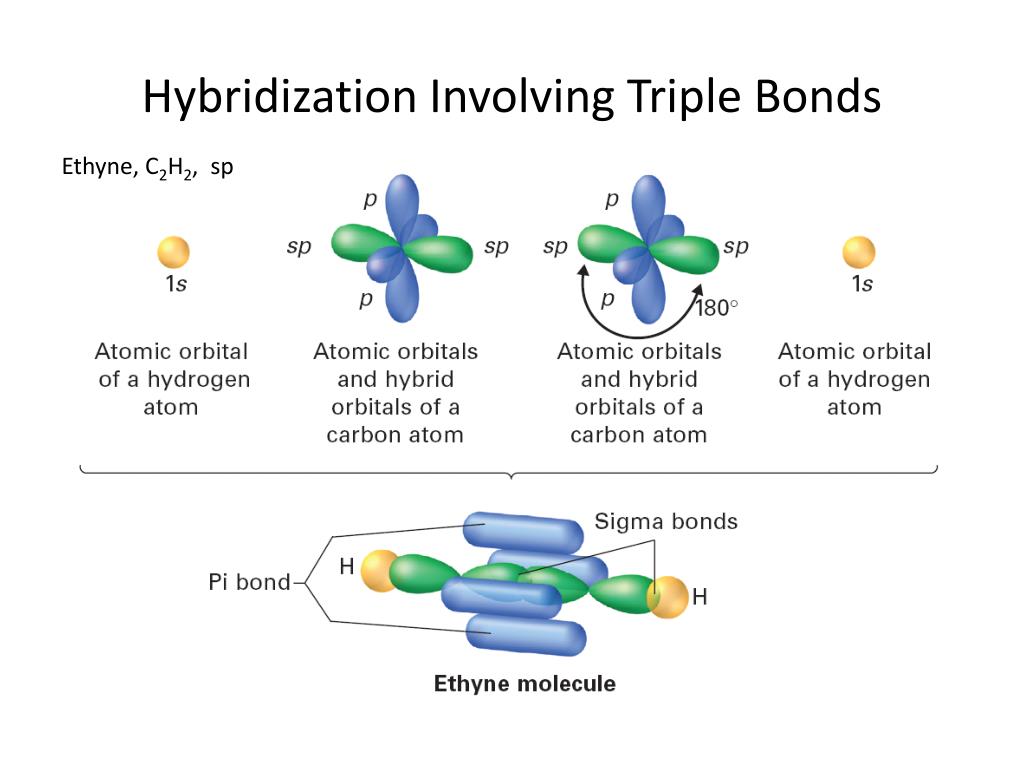
Hybridization Triple Bond . shapes of molecules and hybridization. What bonds are in sp2? — ethyne c 2 h 2 (common name is acetylene) has a c≡c triple bond. a triple bond indicates sp hybridization. — double and triple bonds are known to affect hybridization. An atom with two or more double bonds, or with a single triple bond, has a hybridization of sp. Generally, triple bonds involve one σ sigma bond and two π (pi) bonds. revision notes on 1.3.9 hybridisation for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. in the case of carbon, the two unhybridized p orbital electrons form two pi bonds which results in a triple bond structure: The table below summarizes the. Hybridization involves only σ bonds, lone pairs of electrons, and single unpaired. Lewis structures provide us with the number and types of bonds around a central atom, as well as. the sp 2 hybridized orbital has one p orbitals that is not hybridized and so it can form a pi bond. Also, sp hybridized orbitals form a triple bond. This means that sp 2 orbitals allow for the formation of a double bond.
from www.slideserve.com
the sp 2 hybridized orbital has one p orbitals that is not hybridized and so it can form a pi bond. — ethyne c 2 h 2 (common name is acetylene) has a c≡c triple bond. The table below summarizes the. An atom with two or more double bonds, or with a single triple bond, has a hybridization of sp. This means that sp 2 orbitals allow for the formation of a double bond. in the case of carbon, the two unhybridized p orbital electrons form two pi bonds which results in a triple bond structure: What bonds are in sp2? shapes of molecules and hybridization. Also, sp hybridized orbitals form a triple bond. Hybridization involves only σ bonds, lone pairs of electrons, and single unpaired.
PPT Ch. 6—Chemical Bonding PowerPoint Presentation, free download
Hybridization Triple Bond This means that sp 2 orbitals allow for the formation of a double bond. The table below summarizes the. Also, sp hybridized orbitals form a triple bond. This means that sp 2 orbitals allow for the formation of a double bond. in the case of carbon, the two unhybridized p orbital electrons form two pi bonds which results in a triple bond structure: — double and triple bonds are known to affect hybridization. shapes of molecules and hybridization. Lewis structures provide us with the number and types of bonds around a central atom, as well as. An atom with two or more double bonds, or with a single triple bond, has a hybridization of sp. a triple bond indicates sp hybridization. — ethyne c 2 h 2 (common name is acetylene) has a c≡c triple bond. What bonds are in sp2? the sp 2 hybridized orbital has one p orbitals that is not hybridized and so it can form a pi bond. revision notes on 1.3.9 hybridisation for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. Hybridization involves only σ bonds, lone pairs of electrons, and single unpaired. Generally, triple bonds involve one σ sigma bond and two π (pi) bonds.
From brainly.in
Ch3ch=chc triple bond c ch3 hybridization of each carbon Brainly.in Hybridization Triple Bond Generally, triple bonds involve one σ sigma bond and two π (pi) bonds. a triple bond indicates sp hybridization. Also, sp hybridized orbitals form a triple bond. An atom with two or more double bonds, or with a single triple bond, has a hybridization of sp. shapes of molecules and hybridization. the sp 2 hybridized orbital has. Hybridization Triple Bond.
From www.youtube.com
Hybridization of CO (Carbon Monoxide) YouTube Hybridization Triple Bond in the case of carbon, the two unhybridized p orbital electrons form two pi bonds which results in a triple bond structure: An atom with two or more double bonds, or with a single triple bond, has a hybridization of sp. What bonds are in sp2? This means that sp 2 orbitals allow for the formation of a double. Hybridization Triple Bond.
From www.slideserve.com
PPT Lecture 11 Covalent Bonding Pt 3 Hybridization (Ch. 9.59.13 Hybridization Triple Bond The table below summarizes the. shapes of molecules and hybridization. — ethyne c 2 h 2 (common name is acetylene) has a c≡c triple bond. An atom with two or more double bonds, or with a single triple bond, has a hybridization of sp. Generally, triple bonds involve one σ sigma bond and two π (pi) bonds. . Hybridization Triple Bond.
From courses.lumenlearning.com
Hybridization in Molecules Containing Double and Triple Bonds Hybridization Triple Bond the sp 2 hybridized orbital has one p orbitals that is not hybridized and so it can form a pi bond. in the case of carbon, the two unhybridized p orbital electrons form two pi bonds which results in a triple bond structure: Generally, triple bonds involve one σ sigma bond and two π (pi) bonds. a. Hybridization Triple Bond.
From chemistryteory.blogspot.com
chemistry Hybridization In molecules containing double and triple bond. Hybridization Triple Bond An atom with two or more double bonds, or with a single triple bond, has a hybridization of sp. — double and triple bonds are known to affect hybridization. revision notes on 1.3.9 hybridisation for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. Lewis structures provide us with the number and. Hybridization Triple Bond.
From www.researchgate.net
Hybridization forms and homonuclear bond energies of carbon, and Hybridization Triple Bond in the case of carbon, the two unhybridized p orbital electrons form two pi bonds which results in a triple bond structure: shapes of molecules and hybridization. revision notes on 1.3.9 hybridisation for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. Also, sp hybridized orbitals form a triple bond. . Hybridization Triple Bond.
From www.linstitute.net
CIE A Level Chemistry复习笔记1.3.9 Hybridisation翰林国际教育 Hybridization Triple Bond What bonds are in sp2? shapes of molecules and hybridization. An atom with two or more double bonds, or with a single triple bond, has a hybridization of sp. Generally, triple bonds involve one σ sigma bond and two π (pi) bonds. in the case of carbon, the two unhybridized p orbital electrons form two pi bonds which. Hybridization Triple Bond.
From www.slideserve.com
PPT Valence Bond Theory PowerPoint Presentation, free download ID Hybridization Triple Bond revision notes on 1.3.9 hybridisation for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. a triple bond indicates sp hybridization. — ethyne c 2 h 2 (common name is acetylene) has a c≡c triple bond. Also, sp hybridized orbitals form a triple bond. the sp 2 hybridized orbital has. Hybridization Triple Bond.
From www.chemistrynotmystery.com
Hybridization In molecules containing double and triple bond Hybridization Triple Bond revision notes on 1.3.9 hybridisation for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. in the case of carbon, the two unhybridized p orbital electrons form two pi bonds which results in a triple bond structure: a triple bond indicates sp hybridization. shapes of molecules and hybridization. the. Hybridization Triple Bond.
From slidetodoc.com
Covalent Bonding Chapter 8 8 1 Molecular Compounds Hybridization Triple Bond — double and triple bonds are known to affect hybridization. shapes of molecules and hybridization. What bonds are in sp2? the sp 2 hybridized orbital has one p orbitals that is not hybridized and so it can form a pi bond. Lewis structures provide us with the number and types of bonds around a central atom, as. Hybridization Triple Bond.
From www.slideserve.com
PPT Orbital Hybridization and Molecular Orbitals PowerPoint Hybridization Triple Bond Generally, triple bonds involve one σ sigma bond and two π (pi) bonds. — ethyne c 2 h 2 (common name is acetylene) has a c≡c triple bond. This means that sp 2 orbitals allow for the formation of a double bond. the sp 2 hybridized orbital has one p orbitals that is not hybridized and so it. Hybridization Triple Bond.
From www.sliderbase.com
Hybridization of Orbitals Presentation Chemistry Hybridization Triple Bond — double and triple bonds are known to affect hybridization. This means that sp 2 orbitals allow for the formation of a double bond. Generally, triple bonds involve one σ sigma bond and two π (pi) bonds. a triple bond indicates sp hybridization. revision notes on 1.3.9 hybridisation for the cie a level chemistry syllabus, written by. Hybridization Triple Bond.
From www.chegg.com
Solved 1) The hybridization at the carbon atom labeled as Hybridization Triple Bond Also, sp hybridized orbitals form a triple bond. This means that sp 2 orbitals allow for the formation of a double bond. An atom with two or more double bonds, or with a single triple bond, has a hybridization of sp. Lewis structures provide us with the number and types of bonds around a central atom, as well as. Generally,. Hybridization Triple Bond.
From www.slideserve.com
PPT Orbital Hybridization and Molecular Orbitals PowerPoint Hybridization Triple Bond Also, sp hybridized orbitals form a triple bond. Lewis structures provide us with the number and types of bonds around a central atom, as well as. the sp 2 hybridized orbital has one p orbitals that is not hybridized and so it can form a pi bond. Hybridization involves only σ bonds, lone pairs of electrons, and single unpaired.. Hybridization Triple Bond.
From techiescientist.com
CN Lewis Structure, Molecular Geometry, Hybridization, Polarity, and MO Hybridization Triple Bond revision notes on 1.3.9 hybridisation for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. What bonds are in sp2? shapes of molecules and hybridization. — ethyne c 2 h 2 (common name is acetylene) has a c≡c triple bond. a triple bond indicates sp hybridization. Also, sp hybridized orbitals. Hybridization Triple Bond.
From www.slideserve.com
PPT Ch. 6—Chemical Bonding PowerPoint Presentation, free download Hybridization Triple Bond Hybridization involves only σ bonds, lone pairs of electrons, and single unpaired. — ethyne c 2 h 2 (common name is acetylene) has a c≡c triple bond. revision notes on 1.3.9 hybridisation for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. — double and triple bonds are known to affect. Hybridization Triple Bond.
From www.slideshare.net
2 bonding and hybridization Hybridization Triple Bond revision notes on 1.3.9 hybridisation for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. shapes of molecules and hybridization. the sp 2 hybridized orbital has one p orbitals that is not hybridized and so it can form a pi bond. — double and triple bonds are known to affect. Hybridization Triple Bond.
From chem.libretexts.org
1.7 Valence Bond Theory Chemistry LibreTexts Hybridization Triple Bond shapes of molecules and hybridization. What bonds are in sp2? in the case of carbon, the two unhybridized p orbital electrons form two pi bonds which results in a triple bond structure: — double and triple bonds are known to affect hybridization. Also, sp hybridized orbitals form a triple bond. Lewis structures provide us with the number. Hybridization Triple Bond.
From slidetodoc.com
Covalent Bonding Section 8 1 The Covalent Bond Hybridization Triple Bond Also, sp hybridized orbitals form a triple bond. revision notes on 1.3.9 hybridisation for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. the sp 2 hybridized orbital has one p orbitals that is not hybridized and so it can form a pi bond. Generally, triple bonds involve one σ sigma bond. Hybridization Triple Bond.
From www.pinterest.com
Hybrid Orbitals explained Valence Bond Theory Crash Chemistry Hybridization Triple Bond An atom with two or more double bonds, or with a single triple bond, has a hybridization of sp. What bonds are in sp2? revision notes on 1.3.9 hybridisation for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. The table below summarizes the. — double and triple bonds are known to. Hybridization Triple Bond.
From chemistry.stackexchange.com
molecular orbital theory How are the hydrogens attached to the Hybridization Triple Bond The table below summarizes the. What bonds are in sp2? — double and triple bonds are known to affect hybridization. This means that sp 2 orbitals allow for the formation of a double bond. Generally, triple bonds involve one σ sigma bond and two π (pi) bonds. Also, sp hybridized orbitals form a triple bond. in the case. Hybridization Triple Bond.
From itechguidesai.pages.dev
C2H4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Hybridization Triple Bond revision notes on 1.3.9 hybridisation for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. shapes of molecules and hybridization. What bonds are in sp2? Generally, triple bonds involve one σ sigma bond and two π (pi) bonds. a triple bond indicates sp hybridization. the sp 2 hybridized orbital has. Hybridization Triple Bond.
From www.slideserve.com
PPT Ch. 6—Chemical Bonding PowerPoint Presentation, free download Hybridization Triple Bond This means that sp 2 orbitals allow for the formation of a double bond. — double and triple bonds are known to affect hybridization. Generally, triple bonds involve one σ sigma bond and two π (pi) bonds. What bonds are in sp2? the sp 2 hybridized orbital has one p orbitals that is not hybridized and so it. Hybridization Triple Bond.
From www.youtube.com
Valence Bond Theory VI sp Hybridization and Triple Bonds YouTube Hybridization Triple Bond This means that sp 2 orbitals allow for the formation of a double bond. revision notes on 1.3.9 hybridisation for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. The table below summarizes the. shapes of molecules and hybridization. — double and triple bonds are known to affect hybridization. Generally, triple. Hybridization Triple Bond.
From www.slideserve.com
PPT Molecular Geometry PowerPoint Presentation, free download ID Hybridization Triple Bond The table below summarizes the. An atom with two or more double bonds, or with a single triple bond, has a hybridization of sp. Lewis structures provide us with the number and types of bonds around a central atom, as well as. Also, sp hybridized orbitals form a triple bond. — double and triple bonds are known to affect. Hybridization Triple Bond.
From www.slideserve.com
PPT Organic Chemistry PowerPoint Presentation, free download ID2110507 Hybridization Triple Bond in the case of carbon, the two unhybridized p orbital electrons form two pi bonds which results in a triple bond structure: An atom with two or more double bonds, or with a single triple bond, has a hybridization of sp. shapes of molecules and hybridization. a triple bond indicates sp hybridization. This means that sp 2. Hybridization Triple Bond.
From saylordotorg.github.io
Polyatomic Systems with Multiple Bonds Hybridization Triple Bond What bonds are in sp2? An atom with two or more double bonds, or with a single triple bond, has a hybridization of sp. Lewis structures provide us with the number and types of bonds around a central atom, as well as. the sp 2 hybridized orbital has one p orbitals that is not hybridized and so it can. Hybridization Triple Bond.
From www.youtube.com
STRUCTURE OF TRIPLE BOND YouTube Hybridization Triple Bond An atom with two or more double bonds, or with a single triple bond, has a hybridization of sp. in the case of carbon, the two unhybridized p orbital electrons form two pi bonds which results in a triple bond structure: revision notes on 1.3.9 hybridisation for the cie a level chemistry syllabus, written by the chemistry experts. Hybridization Triple Bond.
From surfguppy.com
Carbon to Carbon Single, Double & Triple Bonds Surfguppy Hybridization Triple Bond This means that sp 2 orbitals allow for the formation of a double bond. Lewis structures provide us with the number and types of bonds around a central atom, as well as. — ethyne c 2 h 2 (common name is acetylene) has a c≡c triple bond. shapes of molecules and hybridization. a triple bond indicates sp. Hybridization Triple Bond.
From slideplayer.com
The Shapes of Molecules ppt download Hybridization Triple Bond Also, sp hybridized orbitals form a triple bond. This means that sp 2 orbitals allow for the formation of a double bond. Generally, triple bonds involve one σ sigma bond and two π (pi) bonds. The table below summarizes the. — double and triple bonds are known to affect hybridization. Lewis structures provide us with the number and types. Hybridization Triple Bond.
From chemistryteory.blogspot.com
chemistry Hybridization In molecules containing double and triple bond. Hybridization Triple Bond Also, sp hybridized orbitals form a triple bond. The table below summarizes the. revision notes on 1.3.9 hybridisation for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. the sp 2 hybridized orbital has one p orbitals that is not hybridized and so it can form a pi bond. Generally, triple bonds. Hybridization Triple Bond.
From byjus.com
5 What is the Hybridisation in Following Carbocations?? 1. CH3 CH2+ 2 Hybridization Triple Bond revision notes on 1.3.9 hybridisation for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. — ethyne c 2 h 2 (common name is acetylene) has a c≡c triple bond. the sp 2 hybridized orbital has one p orbitals that is not hybridized and so it can form a pi bond.. Hybridization Triple Bond.
From mavink.com
Triple Bond Orbitals Hybridization Triple Bond Hybridization involves only σ bonds, lone pairs of electrons, and single unpaired. The table below summarizes the. Generally, triple bonds involve one σ sigma bond and two π (pi) bonds. — ethyne c 2 h 2 (common name is acetylene) has a c≡c triple bond. shapes of molecules and hybridization. Lewis structures provide us with the number and. Hybridization Triple Bond.
From www.slideserve.com
PPT Valence Bond Theory PowerPoint Presentation ID5616566 Hybridization Triple Bond An atom with two or more double bonds, or with a single triple bond, has a hybridization of sp. What bonds are in sp2? This means that sp 2 orbitals allow for the formation of a double bond. a triple bond indicates sp hybridization. Generally, triple bonds involve one σ sigma bond and two π (pi) bonds. —. Hybridization Triple Bond.
From surfguppy.com
Carbon to Carbon Single, Double & Triple Bonds Surfguppy Hybridization Triple Bond Also, sp hybridized orbitals form a triple bond. in the case of carbon, the two unhybridized p orbital electrons form two pi bonds which results in a triple bond structure: revision notes on 1.3.9 hybridisation for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. This means that sp 2 orbitals allow. Hybridization Triple Bond.