Why Do Alkaline Batteries Eventually Stop Working Gcse . Alkaline batteries are non rechargeable. In rechargeable batteries the reactions are reversed by connecting the cells to an external electrical supply. They can be recycled, but need to be replaced. Study with quizlet and memorise flashcards containing terms like why do alkaline batteries eventually stop working?, why can alkaline. Alkaline batteries, like this, eventually run out of stored energy. Alkaline batteries), irreversible reactions take place at the electrodes. This means that electricity can no. Chemical reactions stop when one of the reactants has been used up. After some time, alkaline batteries no longer produce charge. In rechargeable cells and batteries, like the one used to power your mobile phone, the chemical reactions can be reversed when an. Rechargeable batteries, like the battery.
from theconversation.com
Chemical reactions stop when one of the reactants has been used up. Study with quizlet and memorise flashcards containing terms like why do alkaline batteries eventually stop working?, why can alkaline. Alkaline batteries are non rechargeable. Rechargeable batteries, like the battery. Alkaline batteries, like this, eventually run out of stored energy. In rechargeable cells and batteries, like the one used to power your mobile phone, the chemical reactions can be reversed when an. They can be recycled, but need to be replaced. After some time, alkaline batteries no longer produce charge. Alkaline batteries), irreversible reactions take place at the electrodes. This means that electricity can no.
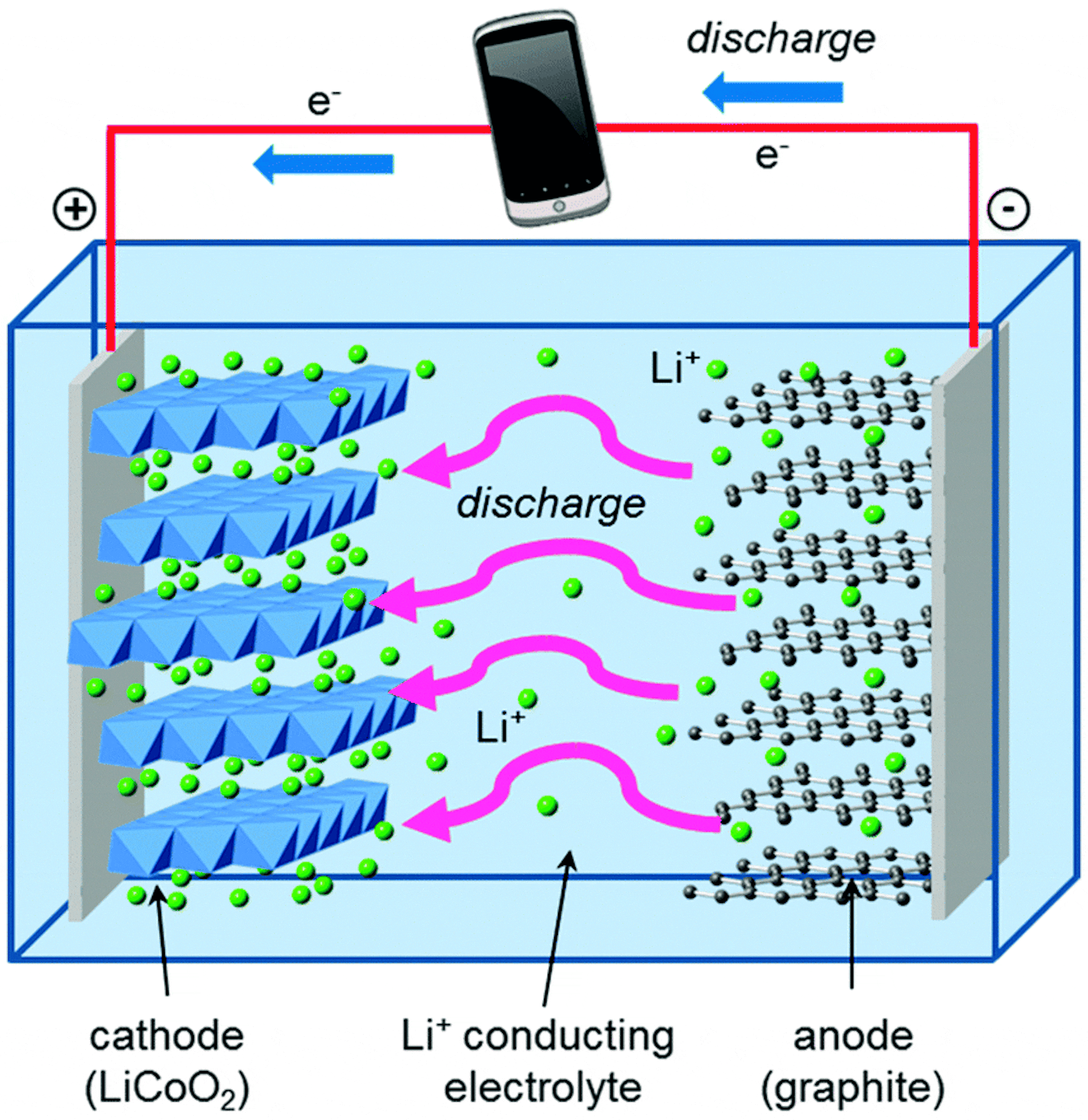
How do lithiumion batteries work?
Why Do Alkaline Batteries Eventually Stop Working Gcse After some time, alkaline batteries no longer produce charge. After some time, alkaline batteries no longer produce charge. Alkaline batteries are non rechargeable. In rechargeable cells and batteries, like the one used to power your mobile phone, the chemical reactions can be reversed when an. Chemical reactions stop when one of the reactants has been used up. Rechargeable batteries, like the battery. Study with quizlet and memorise flashcards containing terms like why do alkaline batteries eventually stop working?, why can alkaline. Alkaline batteries, like this, eventually run out of stored energy. Alkaline batteries), irreversible reactions take place at the electrodes. They can be recycled, but need to be replaced. This means that electricity can no. In rechargeable batteries the reactions are reversed by connecting the cells to an external electrical supply.
From www.livescience.com
Inside Look at How Batteries Work (Infographic) Live Science Why Do Alkaline Batteries Eventually Stop Working Gcse In rechargeable batteries the reactions are reversed by connecting the cells to an external electrical supply. They can be recycled, but need to be replaced. Rechargeable batteries, like the battery. Alkaline batteries are non rechargeable. In rechargeable cells and batteries, like the one used to power your mobile phone, the chemical reactions can be reversed when an. This means that. Why Do Alkaline Batteries Eventually Stop Working Gcse.
From www.scribd.com
Why Should You Use Alkaline Batteries in Your Application? How Does Why Do Alkaline Batteries Eventually Stop Working Gcse Alkaline batteries), irreversible reactions take place at the electrodes. Alkaline batteries, like this, eventually run out of stored energy. This means that electricity can no. Chemical reactions stop when one of the reactants has been used up. After some time, alkaline batteries no longer produce charge. In rechargeable batteries the reactions are reversed by connecting the cells to an external. Why Do Alkaline Batteries Eventually Stop Working Gcse.
From www.slideserve.com
PPT Chapter 21 Electrochemistry PowerPoint Presentation, free Why Do Alkaline Batteries Eventually Stop Working Gcse This means that electricity can no. After some time, alkaline batteries no longer produce charge. They can be recycled, but need to be replaced. Chemical reactions stop when one of the reactants has been used up. Rechargeable batteries, like the battery. Alkaline batteries are non rechargeable. Alkaline batteries, like this, eventually run out of stored energy. Alkaline batteries), irreversible reactions. Why Do Alkaline Batteries Eventually Stop Working Gcse.
From www.youtube.com
Alkaline Batteries Recharging YouTube Why Do Alkaline Batteries Eventually Stop Working Gcse Alkaline batteries, like this, eventually run out of stored energy. After some time, alkaline batteries no longer produce charge. Study with quizlet and memorise flashcards containing terms like why do alkaline batteries eventually stop working?, why can alkaline. This means that electricity can no. They can be recycled, but need to be replaced. Rechargeable batteries, like the battery. In rechargeable. Why Do Alkaline Batteries Eventually Stop Working Gcse.
From courses.lumenlearning.com
Batteries and Fuel Cells Chemistry for Majors Why Do Alkaline Batteries Eventually Stop Working Gcse They can be recycled, but need to be replaced. Alkaline batteries), irreversible reactions take place at the electrodes. This means that electricity can no. Alkaline batteries are non rechargeable. In rechargeable cells and batteries, like the one used to power your mobile phone, the chemical reactions can be reversed when an. Alkaline batteries, like this, eventually run out of stored. Why Do Alkaline Batteries Eventually Stop Working Gcse.
From batteryguy.com
What are Alkaline Batteries? Knowledge Base Why Do Alkaline Batteries Eventually Stop Working Gcse Alkaline batteries), irreversible reactions take place at the electrodes. This means that electricity can no. Study with quizlet and memorise flashcards containing terms like why do alkaline batteries eventually stop working?, why can alkaline. They can be recycled, but need to be replaced. After some time, alkaline batteries no longer produce charge. In rechargeable cells and batteries, like the one. Why Do Alkaline Batteries Eventually Stop Working Gcse.
From www.myfloridahomeenergy.com
Batteries for Home Electronics My Florida Home Energy Why Do Alkaline Batteries Eventually Stop Working Gcse Study with quizlet and memorise flashcards containing terms like why do alkaline batteries eventually stop working?, why can alkaline. Chemical reactions stop when one of the reactants has been used up. In rechargeable cells and batteries, like the one used to power your mobile phone, the chemical reactions can be reversed when an. This means that electricity can no. They. Why Do Alkaline Batteries Eventually Stop Working Gcse.
From www.electricity-magnetism.org
How Batteries Work Basic Principle Electricity Why Do Alkaline Batteries Eventually Stop Working Gcse They can be recycled, but need to be replaced. In rechargeable cells and batteries, like the one used to power your mobile phone, the chemical reactions can be reversed when an. Rechargeable batteries, like the battery. Alkaline batteries are non rechargeable. Chemical reactions stop when one of the reactants has been used up. Alkaline batteries), irreversible reactions take place at. Why Do Alkaline Batteries Eventually Stop Working Gcse.
From www.slideshare.net
Alkaline Battery Why Do Alkaline Batteries Eventually Stop Working Gcse Alkaline batteries), irreversible reactions take place at the electrodes. Alkaline batteries, like this, eventually run out of stored energy. Rechargeable batteries, like the battery. In rechargeable batteries the reactions are reversed by connecting the cells to an external electrical supply. This means that electricity can no. They can be recycled, but need to be replaced. Chemical reactions stop when one. Why Do Alkaline Batteries Eventually Stop Working Gcse.
From www.victorpro.net
Why Are Alkaline Batteries Better Than Other Types? VictorPro Why Do Alkaline Batteries Eventually Stop Working Gcse Alkaline batteries), irreversible reactions take place at the electrodes. Alkaline batteries, like this, eventually run out of stored energy. Chemical reactions stop when one of the reactants has been used up. Rechargeable batteries, like the battery. After some time, alkaline batteries no longer produce charge. In rechargeable batteries the reactions are reversed by connecting the cells to an external electrical. Why Do Alkaline Batteries Eventually Stop Working Gcse.
From byjus.com
How Do Alkaline Batteries Work Structure of Alkaline Battery Why Do Alkaline Batteries Eventually Stop Working Gcse In rechargeable batteries the reactions are reversed by connecting the cells to an external electrical supply. After some time, alkaline batteries no longer produce charge. They can be recycled, but need to be replaced. This means that electricity can no. Study with quizlet and memorise flashcards containing terms like why do alkaline batteries eventually stop working?, why can alkaline. In. Why Do Alkaline Batteries Eventually Stop Working Gcse.
From www.electricity-magnetism.org
Alkaline Battery Description & Application Electricity Why Do Alkaline Batteries Eventually Stop Working Gcse This means that electricity can no. In rechargeable cells and batteries, like the one used to power your mobile phone, the chemical reactions can be reversed when an. Alkaline batteries, like this, eventually run out of stored energy. After some time, alkaline batteries no longer produce charge. Study with quizlet and memorise flashcards containing terms like why do alkaline batteries. Why Do Alkaline Batteries Eventually Stop Working Gcse.
From www.takomabattery.com
The difference between alkaline battery and lead acid battery TYCORUN Why Do Alkaline Batteries Eventually Stop Working Gcse Chemical reactions stop when one of the reactants has been used up. Study with quizlet and memorise flashcards containing terms like why do alkaline batteries eventually stop working?, why can alkaline. They can be recycled, but need to be replaced. Rechargeable batteries, like the battery. Alkaline batteries, like this, eventually run out of stored energy. Alkaline batteries are non rechargeable.. Why Do Alkaline Batteries Eventually Stop Working Gcse.
From powerclues.com
Why are Alkaline Batteries Not Always Recyclable? Power Clues Why Do Alkaline Batteries Eventually Stop Working Gcse Alkaline batteries), irreversible reactions take place at the electrodes. Alkaline batteries, like this, eventually run out of stored energy. Study with quizlet and memorise flashcards containing terms like why do alkaline batteries eventually stop working?, why can alkaline. Rechargeable batteries, like the battery. After some time, alkaline batteries no longer produce charge. This means that electricity can no. In rechargeable. Why Do Alkaline Batteries Eventually Stop Working Gcse.
From www.studypool.com
SOLUTION Gcse electrocells and chemical cells Studypool Why Do Alkaline Batteries Eventually Stop Working Gcse Alkaline batteries are non rechargeable. Alkaline batteries), irreversible reactions take place at the electrodes. In rechargeable batteries the reactions are reversed by connecting the cells to an external electrical supply. This means that electricity can no. Alkaline batteries, like this, eventually run out of stored energy. Rechargeable batteries, like the battery. After some time, alkaline batteries no longer produce charge.. Why Do Alkaline Batteries Eventually Stop Working Gcse.
From www.takomabattery.com
The difference between alkaline battery and lead acid battery TYCORUN Why Do Alkaline Batteries Eventually Stop Working Gcse After some time, alkaline batteries no longer produce charge. Alkaline batteries are non rechargeable. Chemical reactions stop when one of the reactants has been used up. Study with quizlet and memorise flashcards containing terms like why do alkaline batteries eventually stop working?, why can alkaline. Rechargeable batteries, like the battery. In rechargeable batteries the reactions are reversed by connecting the. Why Do Alkaline Batteries Eventually Stop Working Gcse.
From www.howtogeek.com
Why Do Alkaline Batteries Leak? Why Do Alkaline Batteries Eventually Stop Working Gcse This means that electricity can no. In rechargeable batteries the reactions are reversed by connecting the cells to an external electrical supply. Study with quizlet and memorise flashcards containing terms like why do alkaline batteries eventually stop working?, why can alkaline. Chemical reactions stop when one of the reactants has been used up. They can be recycled, but need to. Why Do Alkaline Batteries Eventually Stop Working Gcse.
From www.electricity-magnetism.org
Composition of Alkaline Battery Anode, Cathode & Electrolyte Why Do Alkaline Batteries Eventually Stop Working Gcse In rechargeable cells and batteries, like the one used to power your mobile phone, the chemical reactions can be reversed when an. This means that electricity can no. Rechargeable batteries, like the battery. After some time, alkaline batteries no longer produce charge. They can be recycled, but need to be replaced. Chemical reactions stop when one of the reactants has. Why Do Alkaline Batteries Eventually Stop Working Gcse.
From www.youtube.com
How Does a Battery Work? Alkaline Batteries AP Chemistry YouTube Why Do Alkaline Batteries Eventually Stop Working Gcse In rechargeable batteries the reactions are reversed by connecting the cells to an external electrical supply. Chemical reactions stop when one of the reactants has been used up. After some time, alkaline batteries no longer produce charge. Alkaline batteries, like this, eventually run out of stored energy. They can be recycled, but need to be replaced. This means that electricity. Why Do Alkaline Batteries Eventually Stop Working Gcse.
From www.electricity-magnetism.org
Why are alkaline batteries (AAA or AA) made to be 1.5V while Why Do Alkaline Batteries Eventually Stop Working Gcse Chemical reactions stop when one of the reactants has been used up. In rechargeable batteries the reactions are reversed by connecting the cells to an external electrical supply. Alkaline batteries, like this, eventually run out of stored energy. Rechargeable batteries, like the battery. In rechargeable cells and batteries, like the one used to power your mobile phone, the chemical reactions. Why Do Alkaline Batteries Eventually Stop Working Gcse.
From www.takomabattery.com
The difference between alkaline battery and lead acid battery TYCORUN Why Do Alkaline Batteries Eventually Stop Working Gcse Alkaline batteries are non rechargeable. After some time, alkaline batteries no longer produce charge. They can be recycled, but need to be replaced. Study with quizlet and memorise flashcards containing terms like why do alkaline batteries eventually stop working?, why can alkaline. Alkaline batteries), irreversible reactions take place at the electrodes. Alkaline batteries, like this, eventually run out of stored. Why Do Alkaline Batteries Eventually Stop Working Gcse.
From classnotes.org.in
Batteries Chemistry, Class 12, Electro Chemistry Why Do Alkaline Batteries Eventually Stop Working Gcse After some time, alkaline batteries no longer produce charge. Alkaline batteries), irreversible reactions take place at the electrodes. Alkaline batteries are non rechargeable. In rechargeable batteries the reactions are reversed by connecting the cells to an external electrical supply. Rechargeable batteries, like the battery. Chemical reactions stop when one of the reactants has been used up. This means that electricity. Why Do Alkaline Batteries Eventually Stop Working Gcse.
From www.alwaysreadyhq.com
Why Do Alkaline Batteries Leak? Here's How To Prevent It. Why Do Alkaline Batteries Eventually Stop Working Gcse In rechargeable batteries the reactions are reversed by connecting the cells to an external electrical supply. This means that electricity can no. After some time, alkaline batteries no longer produce charge. Alkaline batteries are non rechargeable. In rechargeable cells and batteries, like the one used to power your mobile phone, the chemical reactions can be reversed when an. Chemical reactions. Why Do Alkaline Batteries Eventually Stop Working Gcse.
From dxogqqbkr.blob.core.windows.net
What Are The Different Types Of Battery Cells at Britteny Chandler blog Why Do Alkaline Batteries Eventually Stop Working Gcse In rechargeable cells and batteries, like the one used to power your mobile phone, the chemical reactions can be reversed when an. Alkaline batteries, like this, eventually run out of stored energy. Study with quizlet and memorise flashcards containing terms like why do alkaline batteries eventually stop working?, why can alkaline. Alkaline batteries are non rechargeable. In rechargeable batteries the. Why Do Alkaline Batteries Eventually Stop Working Gcse.
From exyygeasq.blob.core.windows.net
Why Do Alkaline Batteries Leak at Marguerite Martinez blog Why Do Alkaline Batteries Eventually Stop Working Gcse After some time, alkaline batteries no longer produce charge. Alkaline batteries, like this, eventually run out of stored energy. Rechargeable batteries, like the battery. This means that electricity can no. In rechargeable batteries the reactions are reversed by connecting the cells to an external electrical supply. In rechargeable cells and batteries, like the one used to power your mobile phone,. Why Do Alkaline Batteries Eventually Stop Working Gcse.
From www.slideserve.com
PPT Chemistry 100 Chapter 20 PowerPoint Presentation, free download Why Do Alkaline Batteries Eventually Stop Working Gcse Rechargeable batteries, like the battery. They can be recycled, but need to be replaced. In rechargeable batteries the reactions are reversed by connecting the cells to an external electrical supply. Study with quizlet and memorise flashcards containing terms like why do alkaline batteries eventually stop working?, why can alkaline. In rechargeable cells and batteries, like the one used to power. Why Do Alkaline Batteries Eventually Stop Working Gcse.
From electrical4u.com
Alkaline Batteries Electrical4u Why Do Alkaline Batteries Eventually Stop Working Gcse Rechargeable batteries, like the battery. Alkaline batteries are non rechargeable. After some time, alkaline batteries no longer produce charge. Chemical reactions stop when one of the reactants has been used up. In rechargeable cells and batteries, like the one used to power your mobile phone, the chemical reactions can be reversed when an. Alkaline batteries), irreversible reactions take place at. Why Do Alkaline Batteries Eventually Stop Working Gcse.
From www.verifythis.com
Most AA and AAA batteries can be thrown out in trash Why Do Alkaline Batteries Eventually Stop Working Gcse Study with quizlet and memorise flashcards containing terms like why do alkaline batteries eventually stop working?, why can alkaline. This means that electricity can no. In rechargeable cells and batteries, like the one used to power your mobile phone, the chemical reactions can be reversed when an. Alkaline batteries, like this, eventually run out of stored energy. After some time,. Why Do Alkaline Batteries Eventually Stop Working Gcse.
From mavink.com
Alkaline Dry Cell Battery Diagram Why Do Alkaline Batteries Eventually Stop Working Gcse Study with quizlet and memorise flashcards containing terms like why do alkaline batteries eventually stop working?, why can alkaline. Alkaline batteries), irreversible reactions take place at the electrodes. In rechargeable cells and batteries, like the one used to power your mobile phone, the chemical reactions can be reversed when an. After some time, alkaline batteries no longer produce charge. They. Why Do Alkaline Batteries Eventually Stop Working Gcse.
From courses.lumenlearning.com
Batteries and Fuel Cells Chemistry Atoms First Why Do Alkaline Batteries Eventually Stop Working Gcse After some time, alkaline batteries no longer produce charge. They can be recycled, but need to be replaced. This means that electricity can no. Alkaline batteries, like this, eventually run out of stored energy. Alkaline batteries are non rechargeable. Chemical reactions stop when one of the reactants has been used up. Rechargeable batteries, like the battery. In rechargeable batteries the. Why Do Alkaline Batteries Eventually Stop Working Gcse.
From npplithium.com
Lithium vs Alkaline Batteries, Which is better? What's the Difference Why Do Alkaline Batteries Eventually Stop Working Gcse After some time, alkaline batteries no longer produce charge. Alkaline batteries, like this, eventually run out of stored energy. This means that electricity can no. In rechargeable cells and batteries, like the one used to power your mobile phone, the chemical reactions can be reversed when an. Chemical reactions stop when one of the reactants has been used up. Study. Why Do Alkaline Batteries Eventually Stop Working Gcse.
From theconversation.com
How do lithiumion batteries work? Why Do Alkaline Batteries Eventually Stop Working Gcse Alkaline batteries are non rechargeable. Chemical reactions stop when one of the reactants has been used up. In rechargeable cells and batteries, like the one used to power your mobile phone, the chemical reactions can be reversed when an. This means that electricity can no. Study with quizlet and memorise flashcards containing terms like why do alkaline batteries eventually stop. Why Do Alkaline Batteries Eventually Stop Working Gcse.
From www.xda-developers.com
How does Fast Charging work, and how to use the fastest charging tech Why Do Alkaline Batteries Eventually Stop Working Gcse They can be recycled, but need to be replaced. Alkaline batteries are non rechargeable. After some time, alkaline batteries no longer produce charge. Chemical reactions stop when one of the reactants has been used up. Rechargeable batteries, like the battery. Alkaline batteries), irreversible reactions take place at the electrodes. Study with quizlet and memorise flashcards containing terms like why do. Why Do Alkaline Batteries Eventually Stop Working Gcse.
From thepowerfacts.com
When Was the Alkaline Battery Invented? The Power Facts Why Do Alkaline Batteries Eventually Stop Working Gcse Chemical reactions stop when one of the reactants has been used up. In rechargeable cells and batteries, like the one used to power your mobile phone, the chemical reactions can be reversed when an. Rechargeable batteries, like the battery. This means that electricity can no. After some time, alkaline batteries no longer produce charge. Alkaline batteries), irreversible reactions take place. Why Do Alkaline Batteries Eventually Stop Working Gcse.
From www.micropower-battery.com
How Does an Alkaline Battery Work ? Microcell Battery Why Do Alkaline Batteries Eventually Stop Working Gcse They can be recycled, but need to be replaced. In rechargeable batteries the reactions are reversed by connecting the cells to an external electrical supply. Study with quizlet and memorise flashcards containing terms like why do alkaline batteries eventually stop working?, why can alkaline. Alkaline batteries are non rechargeable. Rechargeable batteries, like the battery. Alkaline batteries), irreversible reactions take place. Why Do Alkaline Batteries Eventually Stop Working Gcse.