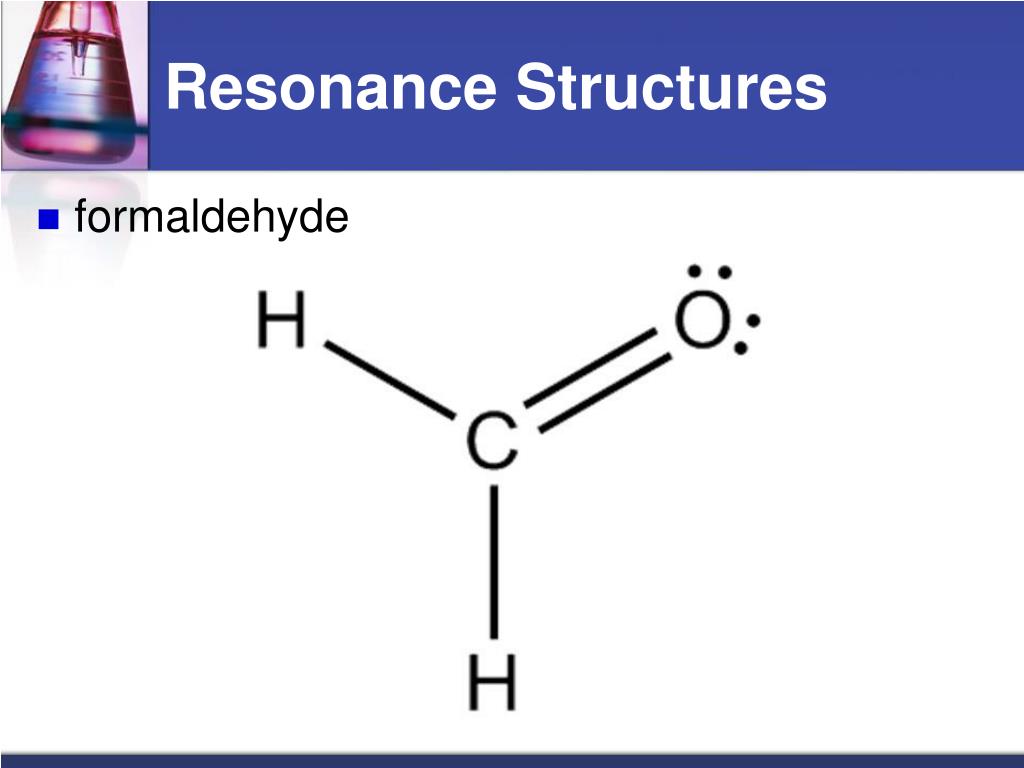
Formaldehyde Resonance Structures . the resonance structures in figure 1 illustrate this polarity, and the relative dipole moments of formaldehyde, other. ch 2 o has resonance structures, which means that the compound’s single lewis structure is unable to explain all the bonding in the molecule. the lewis structure of formaldehyde, ch 2 o, that contributes the most to the bonding in the molecule is as follows: Lewis diagram of formaldehyde (ch₂o) the lewis diagram of. Rules for drawing resonance structures 1. Recognizing, drawing, and evaluating the relative. Looking at the structure of formaldehyde. rules for drawing and working with resonance contributors.
from www.slideserve.com
Recognizing, drawing, and evaluating the relative. ch 2 o has resonance structures, which means that the compound’s single lewis structure is unable to explain all the bonding in the molecule. the lewis structure of formaldehyde, ch 2 o, that contributes the most to the bonding in the molecule is as follows: Rules for drawing resonance structures 1. the resonance structures in figure 1 illustrate this polarity, and the relative dipole moments of formaldehyde, other. rules for drawing and working with resonance contributors. Lewis diagram of formaldehyde (ch₂o) the lewis diagram of. Looking at the structure of formaldehyde.
PPT Chapter 2 PowerPoint Presentation, free download ID5950124
Formaldehyde Resonance Structures rules for drawing and working with resonance contributors. rules for drawing and working with resonance contributors. Lewis diagram of formaldehyde (ch₂o) the lewis diagram of. Rules for drawing resonance structures 1. Looking at the structure of formaldehyde. the lewis structure of formaldehyde, ch 2 o, that contributes the most to the bonding in the molecule is as follows: the resonance structures in figure 1 illustrate this polarity, and the relative dipole moments of formaldehyde, other. ch 2 o has resonance structures, which means that the compound’s single lewis structure is unable to explain all the bonding in the molecule. Recognizing, drawing, and evaluating the relative.
From solvedlib.com
Given the Lewis structure of formaldehyde (CH2O) sho… SolvedLib Formaldehyde Resonance Structures Lewis diagram of formaldehyde (ch₂o) the lewis diagram of. Rules for drawing resonance structures 1. the resonance structures in figure 1 illustrate this polarity, and the relative dipole moments of formaldehyde, other. Looking at the structure of formaldehyde. the lewis structure of formaldehyde, ch 2 o, that contributes the most to the bonding in the molecule is as. Formaldehyde Resonance Structures.
From www.chegg.com
Solved Which of the following represents the correct Formaldehyde Resonance Structures the resonance structures in figure 1 illustrate this polarity, and the relative dipole moments of formaldehyde, other. Looking at the structure of formaldehyde. Lewis diagram of formaldehyde (ch₂o) the lewis diagram of. the lewis structure of formaldehyde, ch 2 o, that contributes the most to the bonding in the molecule is as follows: ch 2 o has. Formaldehyde Resonance Structures.
From www.researchgate.net
Structure of formaldehyde [5] Download Scientific Diagram Formaldehyde Resonance Structures rules for drawing and working with resonance contributors. the lewis structure of formaldehyde, ch 2 o, that contributes the most to the bonding in the molecule is as follows: Recognizing, drawing, and evaluating the relative. the resonance structures in figure 1 illustrate this polarity, and the relative dipole moments of formaldehyde, other. Looking at the structure of. Formaldehyde Resonance Structures.
From www.youtube.com
H2CO Lewis Structure (Formaldehyde) YouTube Formaldehyde Resonance Structures rules for drawing and working with resonance contributors. the resonance structures in figure 1 illustrate this polarity, and the relative dipole moments of formaldehyde, other. Looking at the structure of formaldehyde. Lewis diagram of formaldehyde (ch₂o) the lewis diagram of. Rules for drawing resonance structures 1. the lewis structure of formaldehyde, ch 2 o, that contributes the. Formaldehyde Resonance Structures.
From www.coursehero.com
[Solved] What will be the charge of the ion formed from each of these Formaldehyde Resonance Structures the resonance structures in figure 1 illustrate this polarity, and the relative dipole moments of formaldehyde, other. Recognizing, drawing, and evaluating the relative. rules for drawing and working with resonance contributors. Looking at the structure of formaldehyde. Rules for drawing resonance structures 1. ch 2 o has resonance structures, which means that the compound’s single lewis structure. Formaldehyde Resonance Structures.
From www.animalia-life.club
Formaldehyde Molecular Geometry Formaldehyde Resonance Structures the lewis structure of formaldehyde, ch 2 o, that contributes the most to the bonding in the molecule is as follows: the resonance structures in figure 1 illustrate this polarity, and the relative dipole moments of formaldehyde, other. ch 2 o has resonance structures, which means that the compound’s single lewis structure is unable to explain all. Formaldehyde Resonance Structures.
From www.slideserve.com
PPT Chapter 2 PowerPoint Presentation, free download ID5950124 Formaldehyde Resonance Structures Recognizing, drawing, and evaluating the relative. the lewis structure of formaldehyde, ch 2 o, that contributes the most to the bonding in the molecule is as follows: Looking at the structure of formaldehyde. ch 2 o has resonance structures, which means that the compound’s single lewis structure is unable to explain all the bonding in the molecule. . Formaldehyde Resonance Structures.
From www.measuringknowhow.com
Understanding the R Effect What Is It and Why Does It Matter Formaldehyde Resonance Structures Looking at the structure of formaldehyde. rules for drawing and working with resonance contributors. ch 2 o has resonance structures, which means that the compound’s single lewis structure is unable to explain all the bonding in the molecule. the lewis structure of formaldehyde, ch 2 o, that contributes the most to the bonding in the molecule is. Formaldehyde Resonance Structures.
From proper-cooking.info
Formaldehyde Lewis Structure Resonance Formaldehyde Resonance Structures Lewis diagram of formaldehyde (ch₂o) the lewis diagram of. Looking at the structure of formaldehyde. the resonance structures in figure 1 illustrate this polarity, and the relative dipole moments of formaldehyde, other. rules for drawing and working with resonance contributors. ch 2 o has resonance structures, which means that the compound’s single lewis structure is unable to. Formaldehyde Resonance Structures.
From www.organicchemistrytutor.com
Lewis Structures — Organic Chemistry Tutor Formaldehyde Resonance Structures rules for drawing and working with resonance contributors. ch 2 o has resonance structures, which means that the compound’s single lewis structure is unable to explain all the bonding in the molecule. Rules for drawing resonance structures 1. Looking at the structure of formaldehyde. Recognizing, drawing, and evaluating the relative. Lewis diagram of formaldehyde (ch₂o) the lewis diagram. Formaldehyde Resonance Structures.
From ar.inspiredpencil.com
Formaldehyde Lewis Structure Resonance Formaldehyde Resonance Structures Recognizing, drawing, and evaluating the relative. Lewis diagram of formaldehyde (ch₂o) the lewis diagram of. Rules for drawing resonance structures 1. rules for drawing and working with resonance contributors. ch 2 o has resonance structures, which means that the compound’s single lewis structure is unable to explain all the bonding in the molecule. the lewis structure of. Formaldehyde Resonance Structures.
From www.dreamstime.com
3D Image of Formaldehyde Skeletal Formula Stock Illustration Formaldehyde Resonance Structures rules for drawing and working with resonance contributors. Looking at the structure of formaldehyde. the resonance structures in figure 1 illustrate this polarity, and the relative dipole moments of formaldehyde, other. Lewis diagram of formaldehyde (ch₂o) the lewis diagram of. ch 2 o has resonance structures, which means that the compound’s single lewis structure is unable to. Formaldehyde Resonance Structures.
From www.youtube.com
H2CO (Formaldehyde) Molecular Geometry, Bond Angles (and Electron Formaldehyde Resonance Structures Rules for drawing resonance structures 1. ch 2 o has resonance structures, which means that the compound’s single lewis structure is unable to explain all the bonding in the molecule. rules for drawing and working with resonance contributors. Looking at the structure of formaldehyde. Recognizing, drawing, and evaluating the relative. Lewis diagram of formaldehyde (ch₂o) the lewis diagram. Formaldehyde Resonance Structures.
From www.animalia-life.club
Formaldehyde Molecular Geometry Formaldehyde Resonance Structures Looking at the structure of formaldehyde. Rules for drawing resonance structures 1. rules for drawing and working with resonance contributors. the resonance structures in figure 1 illustrate this polarity, and the relative dipole moments of formaldehyde, other. the lewis structure of formaldehyde, ch 2 o, that contributes the most to the bonding in the molecule is as. Formaldehyde Resonance Structures.
From ar.inspiredpencil.com
Formaldehyde Lewis Structure Resonance Formaldehyde Resonance Structures rules for drawing and working with resonance contributors. Lewis diagram of formaldehyde (ch₂o) the lewis diagram of. ch 2 o has resonance structures, which means that the compound’s single lewis structure is unable to explain all the bonding in the molecule. Looking at the structure of formaldehyde. Recognizing, drawing, and evaluating the relative. the resonance structures in. Formaldehyde Resonance Structures.
From www.vrogue.co
Draw The Lewis Dot Structure Of Formaldehyde Quizlet vrogue.co Formaldehyde Resonance Structures rules for drawing and working with resonance contributors. Rules for drawing resonance structures 1. the resonance structures in figure 1 illustrate this polarity, and the relative dipole moments of formaldehyde, other. Recognizing, drawing, and evaluating the relative. the lewis structure of formaldehyde, ch 2 o, that contributes the most to the bonding in the molecule is as. Formaldehyde Resonance Structures.
From www.youtube.com
Structure and Uses of Formaldehyde and Paraldehyde YouTube Formaldehyde Resonance Structures Lewis diagram of formaldehyde (ch₂o) the lewis diagram of. the resonance structures in figure 1 illustrate this polarity, and the relative dipole moments of formaldehyde, other. the lewis structure of formaldehyde, ch 2 o, that contributes the most to the bonding in the molecule is as follows: Recognizing, drawing, and evaluating the relative. Looking at the structure of. Formaldehyde Resonance Structures.
From www.youtube.com
How to Draw the Lewis Dot Structure for CH2O Formaldehyde YouTube Formaldehyde Resonance Structures Recognizing, drawing, and evaluating the relative. the lewis structure of formaldehyde, ch 2 o, that contributes the most to the bonding in the molecule is as follows: Rules for drawing resonance structures 1. ch 2 o has resonance structures, which means that the compound’s single lewis structure is unable to explain all the bonding in the molecule. Looking. Formaldehyde Resonance Structures.
From ar.inspiredpencil.com
Formaldehyde Lewis Structure Resonance Formaldehyde Resonance Structures Lewis diagram of formaldehyde (ch₂o) the lewis diagram of. Looking at the structure of formaldehyde. rules for drawing and working with resonance contributors. the resonance structures in figure 1 illustrate this polarity, and the relative dipole moments of formaldehyde, other. Rules for drawing resonance structures 1. ch 2 o has resonance structures, which means that the compound’s. Formaldehyde Resonance Structures.
From www.shutterstock.com
Formaldehyde Ch2o Molecule Chemical Structure Stock Illustration Formaldehyde Resonance Structures the lewis structure of formaldehyde, ch 2 o, that contributes the most to the bonding in the molecule is as follows: Lewis diagram of formaldehyde (ch₂o) the lewis diagram of. Looking at the structure of formaldehyde. the resonance structures in figure 1 illustrate this polarity, and the relative dipole moments of formaldehyde, other. rules for drawing and. Formaldehyde Resonance Structures.
From www.slideserve.com
PPT Chapter 2 PowerPoint Presentation, free download ID5950124 Formaldehyde Resonance Structures Lewis diagram of formaldehyde (ch₂o) the lewis diagram of. the resonance structures in figure 1 illustrate this polarity, and the relative dipole moments of formaldehyde, other. the lewis structure of formaldehyde, ch 2 o, that contributes the most to the bonding in the molecule is as follows: Looking at the structure of formaldehyde. ch 2 o has. Formaldehyde Resonance Structures.
From ar.inspiredpencil.com
Formaldehyde Lewis Structure Resonance Formaldehyde Resonance Structures Recognizing, drawing, and evaluating the relative. the resonance structures in figure 1 illustrate this polarity, and the relative dipole moments of formaldehyde, other. ch 2 o has resonance structures, which means that the compound’s single lewis structure is unable to explain all the bonding in the molecule. Looking at the structure of formaldehyde. Rules for drawing resonance structures. Formaldehyde Resonance Structures.
From www.youtube.com
Worked example Lewis diagram of formaldehyde (CH₂O) AP Chemistry Formaldehyde Resonance Structures Looking at the structure of formaldehyde. Lewis diagram of formaldehyde (ch₂o) the lewis diagram of. rules for drawing and working with resonance contributors. ch 2 o has resonance structures, which means that the compound’s single lewis structure is unable to explain all the bonding in the molecule. the lewis structure of formaldehyde, ch 2 o, that contributes. Formaldehyde Resonance Structures.
From www.semanticscholar.org
[PDF] Effect of different catalysts on ureaformaldehyde resin Formaldehyde Resonance Structures the resonance structures in figure 1 illustrate this polarity, and the relative dipole moments of formaldehyde, other. Lewis diagram of formaldehyde (ch₂o) the lewis diagram of. Rules for drawing resonance structures 1. Looking at the structure of formaldehyde. the lewis structure of formaldehyde, ch 2 o, that contributes the most to the bonding in the molecule is as. Formaldehyde Resonance Structures.
From www.mdpi.com
Life Free FullText Formaldehyde—A Key Monad of the Biomolecular System Formaldehyde Resonance Structures Looking at the structure of formaldehyde. the resonance structures in figure 1 illustrate this polarity, and the relative dipole moments of formaldehyde, other. the lewis structure of formaldehyde, ch 2 o, that contributes the most to the bonding in the molecule is as follows: Rules for drawing resonance structures 1. Lewis diagram of formaldehyde (ch₂o) the lewis diagram. Formaldehyde Resonance Structures.
From www.dreamstime.com
Formaldehyde Molecular Structure 3D Icon Stock Vector Illustration Formaldehyde Resonance Structures Looking at the structure of formaldehyde. Lewis diagram of formaldehyde (ch₂o) the lewis diagram of. Recognizing, drawing, and evaluating the relative. Rules for drawing resonance structures 1. the resonance structures in figure 1 illustrate this polarity, and the relative dipole moments of formaldehyde, other. rules for drawing and working with resonance contributors. the lewis structure of formaldehyde,. Formaldehyde Resonance Structures.
From www.semanticscholar.org
[PDF] Effect of different catalysts on ureaformaldehyde resin Formaldehyde Resonance Structures rules for drawing and working with resonance contributors. Looking at the structure of formaldehyde. Lewis diagram of formaldehyde (ch₂o) the lewis diagram of. ch 2 o has resonance structures, which means that the compound’s single lewis structure is unable to explain all the bonding in the molecule. Recognizing, drawing, and evaluating the relative. Rules for drawing resonance structures. Formaldehyde Resonance Structures.
From courses.lumenlearning.com
6.2. Resonance Organic Chemistry 1 An open textbook Formaldehyde Resonance Structures the lewis structure of formaldehyde, ch 2 o, that contributes the most to the bonding in the molecule is as follows: rules for drawing and working with resonance contributors. the resonance structures in figure 1 illustrate this polarity, and the relative dipole moments of formaldehyde, other. Lewis diagram of formaldehyde (ch₂o) the lewis diagram of. Recognizing, drawing,. Formaldehyde Resonance Structures.
From chem2401.wordpress.com
How to Draw Resonance Structures Organic Chemistry Formaldehyde Resonance Structures Recognizing, drawing, and evaluating the relative. the resonance structures in figure 1 illustrate this polarity, and the relative dipole moments of formaldehyde, other. rules for drawing and working with resonance contributors. Rules for drawing resonance structures 1. the lewis structure of formaldehyde, ch 2 o, that contributes the most to the bonding in the molecule is as. Formaldehyde Resonance Structures.
From www.pinterest.com
Draw a Lewis Structure of Formaldehyde in 2020 Molecular geometry Formaldehyde Resonance Structures the lewis structure of formaldehyde, ch 2 o, that contributes the most to the bonding in the molecule is as follows: Recognizing, drawing, and evaluating the relative. Lewis diagram of formaldehyde (ch₂o) the lewis diagram of. the resonance structures in figure 1 illustrate this polarity, and the relative dipole moments of formaldehyde, other. ch 2 o has. Formaldehyde Resonance Structures.
From www.slideserve.com
PPT Structure and Bonding in Organic Chemistry PowerPoint Formaldehyde Resonance Structures the lewis structure of formaldehyde, ch 2 o, that contributes the most to the bonding in the molecule is as follows: the resonance structures in figure 1 illustrate this polarity, and the relative dipole moments of formaldehyde, other. ch 2 o has resonance structures, which means that the compound’s single lewis structure is unable to explain all. Formaldehyde Resonance Structures.
From chem.libretexts.org
19.5 Nucleophilic Addition Reactions of Aldehydes and Ketones Formaldehyde Resonance Structures Lewis diagram of formaldehyde (ch₂o) the lewis diagram of. rules for drawing and working with resonance contributors. the lewis structure of formaldehyde, ch 2 o, that contributes the most to the bonding in the molecule is as follows: Looking at the structure of formaldehyde. the resonance structures in figure 1 illustrate this polarity, and the relative dipole. Formaldehyde Resonance Structures.
From mugeek.vidalondon.net
Chemical Makeup Of Formaldehyde Mugeek Vidalondon Formaldehyde Resonance Structures Rules for drawing resonance structures 1. ch 2 o has resonance structures, which means that the compound’s single lewis structure is unable to explain all the bonding in the molecule. the lewis structure of formaldehyde, ch 2 o, that contributes the most to the bonding in the molecule is as follows: Lewis diagram of formaldehyde (ch₂o) the lewis. Formaldehyde Resonance Structures.
From www.researchgate.net
(A) Reaction of formaldehyde with DNPH to form stable hydrazone. (B Formaldehyde Resonance Structures Looking at the structure of formaldehyde. the lewis structure of formaldehyde, ch 2 o, that contributes the most to the bonding in the molecule is as follows: Lewis diagram of formaldehyde (ch₂o) the lewis diagram of. the resonance structures in figure 1 illustrate this polarity, and the relative dipole moments of formaldehyde, other. Recognizing, drawing, and evaluating the. Formaldehyde Resonance Structures.
From chemicaldb.netlify.app
Formaldehyde electrolyte type Formaldehyde Resonance Structures the resonance structures in figure 1 illustrate this polarity, and the relative dipole moments of formaldehyde, other. rules for drawing and working with resonance contributors. Recognizing, drawing, and evaluating the relative. Looking at the structure of formaldehyde. ch 2 o has resonance structures, which means that the compound’s single lewis structure is unable to explain all the. Formaldehyde Resonance Structures.