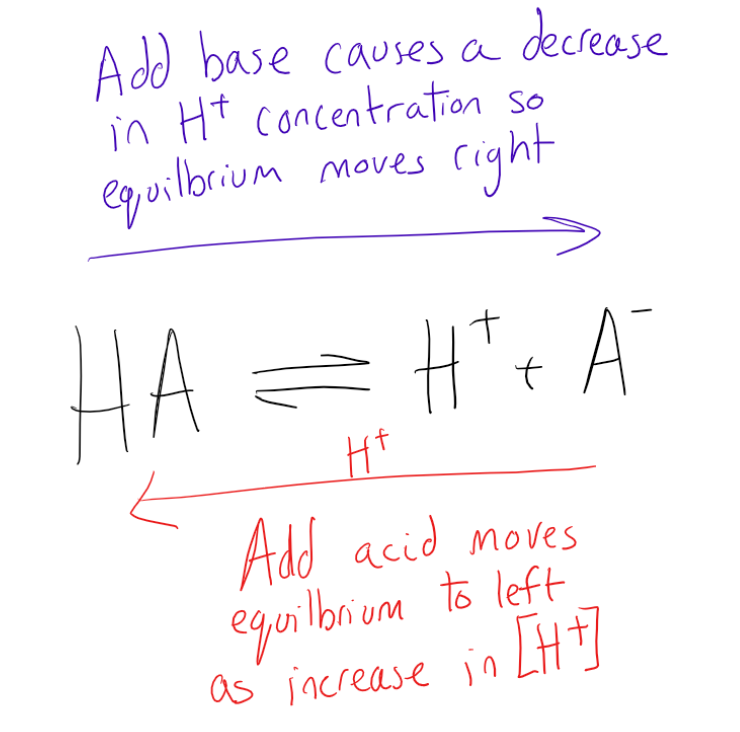
Adding Base To Buffer Solution . It is able to neutralize small amounts of added acid or base, thus. A buffer solution is one which resists changes in ph. This page describes simple acidic and alkaline buffer solutions and explains how they work. The buffer solution is a solution able to maintain its hydrogen ion concentration (ph) with only minor changes in the dilution or addition of a. Amount of an acid or base that can be added to a volume of a buffer solution before its ph changes significantly (usually by one ph unit); A buffer solution consists of a weak acid and its conjugate base or a. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. The buffer is extremely effective at resisting a change in ph because the added hydroxide ion attacks the weak acid (in very high. In other words, the amount of acid or base a buffer. An aqueous solution that resists any change in ph by adding a small amount of acid or base is called a buffer solution. What is a buffer solution? A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or.
from jackwestin.com
A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer solution consists of a weak acid and its conjugate base or a. This page describes simple acidic and alkaline buffer solutions and explains how they work. In other words, the amount of acid or base a buffer. What is a buffer solution? The buffer is extremely effective at resisting a change in ph because the added hydroxide ion attacks the weak acid (in very high. Amount of an acid or base that can be added to a volume of a buffer solution before its ph changes significantly (usually by one ph unit); It is able to neutralize small amounts of added acid or base, thus. A buffer solution is one which resists changes in ph.
Buffers 2 Acid Base Equilibria MCAT Content
Adding Base To Buffer Solution A buffer solution is one which resists changes in ph. An aqueous solution that resists any change in ph by adding a small amount of acid or base is called a buffer solution. In other words, the amount of acid or base a buffer. The buffer solution is a solution able to maintain its hydrogen ion concentration (ph) with only minor changes in the dilution or addition of a. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. The buffer is extremely effective at resisting a change in ph because the added hydroxide ion attacks the weak acid (in very high. It is able to neutralize small amounts of added acid or base, thus. Amount of an acid or base that can be added to a volume of a buffer solution before its ph changes significantly (usually by one ph unit); A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. This page describes simple acidic and alkaline buffer solutions and explains how they work. A buffer solution is one which resists changes in ph. What is a buffer solution? A buffer solution consists of a weak acid and its conjugate base or a.
From studylib.net
Buffer Solutions Adding Base To Buffer Solution What is a buffer solution? It is able to neutralize small amounts of added acid or base, thus. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. Amount of an acid or base that can be added to a volume of a. Adding Base To Buffer Solution.
From www.chegg.com
Solved A buffer is a solution that is a mixture of either a Adding Base To Buffer Solution The buffer solution is a solution able to maintain its hydrogen ion concentration (ph) with only minor changes in the dilution or addition of a. A buffer solution consists of a weak acid and its conjugate base or a. This page describes simple acidic and alkaline buffer solutions and explains how they work. Amount of an acid or base that. Adding Base To Buffer Solution.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG Adding Base To Buffer Solution A buffer solution is one which resists changes in ph. What is a buffer solution? A buffer solution consists of a weak acid and its conjugate base or a. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. The buffer solution is. Adding Base To Buffer Solution.
From www.slideserve.com
PPT DEFINITIONS OF ACIDS & BASES PowerPoint Presentation, free Adding Base To Buffer Solution The buffer solution is a solution able to maintain its hydrogen ion concentration (ph) with only minor changes in the dilution or addition of a. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. A buffer is a solution that can resist. Adding Base To Buffer Solution.
From www.slideserve.com
PPT Chemistry 100 Chapter 17 PowerPoint Presentation, free download Adding Base To Buffer Solution A buffer solution is one which resists changes in ph. This page describes simple acidic and alkaline buffer solutions and explains how they work. The buffer is extremely effective at resisting a change in ph because the added hydroxide ion attacks the weak acid (in very high. A buffer is a solution that can resist ph change upon the addition. Adding Base To Buffer Solution.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint Adding Base To Buffer Solution In other words, the amount of acid or base a buffer. Amount of an acid or base that can be added to a volume of a buffer solution before its ph changes significantly (usually by one ph unit); It is able to neutralize small amounts of added acid or base, thus. An aqueous solution that resists any change in ph. Adding Base To Buffer Solution.
From www.slideserve.com
PPT Chemistry 100 Chapter 17 PowerPoint Presentation, free download Adding Base To Buffer Solution This page describes simple acidic and alkaline buffer solutions and explains how they work. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. In other words, the amount of acid or base a buffer. It is able to neutralize small amounts of added acid or base, thus. An aqueous solution. Adding Base To Buffer Solution.
From www.brainkart.com
How do buffers work? Adding Base To Buffer Solution A buffer solution consists of a weak acid and its conjugate base or a. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. An aqueous solution that resists any change in ph by adding a small amount of acid or base is. Adding Base To Buffer Solution.
From www.slideshare.net
2012 topic 18 2 buffer solutions Adding Base To Buffer Solution Amount of an acid or base that can be added to a volume of a buffer solution before its ph changes significantly (usually by one ph unit); A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. The buffer solution is a solution. Adding Base To Buffer Solution.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID6787517 Adding Base To Buffer Solution What is a buffer solution? Amount of an acid or base that can be added to a volume of a buffer solution before its ph changes significantly (usually by one ph unit); The buffer is extremely effective at resisting a change in ph because the added hydroxide ion attacks the weak acid (in very high. A mixture of a weak. Adding Base To Buffer Solution.
From www.pinterest.es
Understanding Buffer Solution Chemistry A Complete Guide Adding Base To Buffer Solution This page describes simple acidic and alkaline buffer solutions and explains how they work. The buffer is extremely effective at resisting a change in ph because the added hydroxide ion attacks the weak acid (in very high. What is a buffer solution? A buffer solution consists of a weak acid and its conjugate base or a. It is able to. Adding Base To Buffer Solution.
From jackwestin.com
Buffers 2 Acid Base Equilibria MCAT Content Adding Base To Buffer Solution A buffer solution is one which resists changes in ph. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. The buffer solution is a solution able to maintain its hydrogen ion concentration (ph) with only minor changes in the dilution or addition of a. In other words, the amount of. Adding Base To Buffer Solution.
From www.chemistrylearner.com
Buffer Solution Definition, Examples, and Preparation Adding Base To Buffer Solution This page describes simple acidic and alkaline buffer solutions and explains how they work. The buffer solution is a solution able to maintain its hydrogen ion concentration (ph) with only minor changes in the dilution or addition of a. In other words, the amount of acid or base a buffer. An aqueous solution that resists any change in ph by. Adding Base To Buffer Solution.
From www.slideserve.com
PPT Chapter 17 Additional Aspects of Aqueous Equilibria PowerPoint Adding Base To Buffer Solution Amount of an acid or base that can be added to a volume of a buffer solution before its ph changes significantly (usually by one ph unit); A buffer solution is one which resists changes in ph. A buffer solution consists of a weak acid and its conjugate base or a. A mixture of a weak acid and its conjugate. Adding Base To Buffer Solution.
From eumeducationkr.com
YoutubePosterImageILTACollectionChemistryAddingStrongBaseto Adding Base To Buffer Solution The buffer solution is a solution able to maintain its hydrogen ion concentration (ph) with only minor changes in the dilution or addition of a. What is a buffer solution? A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. In other words,. Adding Base To Buffer Solution.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint Adding Base To Buffer Solution In other words, the amount of acid or base a buffer. This page describes simple acidic and alkaline buffer solutions and explains how they work. What is a buffer solution? A buffer solution consists of a weak acid and its conjugate base or a. A buffer is a solution that can resist ph change upon the addition of an acidic. Adding Base To Buffer Solution.
From slideplayer.com
SCH4U Acids and Bases BUFFER SOLUTIONS. ppt download Adding Base To Buffer Solution This page describes simple acidic and alkaline buffer solutions and explains how they work. It is able to neutralize small amounts of added acid or base, thus. An aqueous solution that resists any change in ph by adding a small amount of acid or base is called a buffer solution. In other words, the amount of acid or base a. Adding Base To Buffer Solution.
From criticalthinking.cloud
how to solve buffer problems chemistry Adding Base To Buffer Solution It is able to neutralize small amounts of added acid or base, thus. Amount of an acid or base that can be added to a volume of a buffer solution before its ph changes significantly (usually by one ph unit); A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. The. Adding Base To Buffer Solution.
From chem.libretexts.org
8.8 Buffers Solutions That Resist pH Change Chemistry LibreTexts Adding Base To Buffer Solution This page describes simple acidic and alkaline buffer solutions and explains how they work. What is a buffer solution? A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. In other words, the amount of acid or base a buffer. Amount of an. Adding Base To Buffer Solution.
From courses.lumenlearning.com
Buffers Chemistry for Majors Adding Base To Buffer Solution The buffer is extremely effective at resisting a change in ph because the added hydroxide ion attacks the weak acid (in very high. This page describes simple acidic and alkaline buffer solutions and explains how they work. A buffer solution is one which resists changes in ph. A mixture of a weak acid and its conjugate base (or a mixture. Adding Base To Buffer Solution.
From www.youtube.com
Acids and Bases Buffer Calculation Past Paper Exam Question Adding Base To Buffer Solution An aqueous solution that resists any change in ph by adding a small amount of acid or base is called a buffer solution. A buffer solution consists of a weak acid and its conjugate base or a. In other words, the amount of acid or base a buffer. It is able to neutralize small amounts of added acid or base,. Adding Base To Buffer Solution.
From skc13chem.blogspot.com
SKC year 13 Chemistry Buffer solutions Adding Base To Buffer Solution In other words, the amount of acid or base a buffer. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. This page describes simple acidic and alkaline buffer solutions and explains how they work. A mixture of a weak acid and its conjugate base (or a mixture of a weak. Adding Base To Buffer Solution.
From allyson-has-mccoy.blogspot.com
Which Pair Will Produce a Buffer Solution AllysonhasMccoy Adding Base To Buffer Solution The buffer solution is a solution able to maintain its hydrogen ion concentration (ph) with only minor changes in the dilution or addition of a. What is a buffer solution? A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize small amounts of added acid or. Adding Base To Buffer Solution.
From classnotes.org.in
Buffer solution and Buffer Action Chemistry, Class 11, Ionic Equilibrium Adding Base To Buffer Solution In other words, the amount of acid or base a buffer. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. This page describes simple acidic and alkaline buffer solutions and explains how they work. A buffer solution is one which resists changes in ph. Amount of an acid or base. Adding Base To Buffer Solution.
From www.slideserve.com
PPT Today’s Objective SWBAT explain how a buffer works. SWBAT Adding Base To Buffer Solution The buffer solution is a solution able to maintain its hydrogen ion concentration (ph) with only minor changes in the dilution or addition of a. Amount of an acid or base that can be added to a volume of a buffer solution before its ph changes significantly (usually by one ph unit); In other words, the amount of acid or. Adding Base To Buffer Solution.
From www.youtube.com
pH of buffer solutions the HendersonHasselbalch equation. YouTube Adding Base To Buffer Solution An aqueous solution that resists any change in ph by adding a small amount of acid or base is called a buffer solution. A buffer solution consists of a weak acid and its conjugate base or a. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A mixture of a. Adding Base To Buffer Solution.
From www.youtube.com
Adding strong base to a buffer solution YouTube Adding Base To Buffer Solution A buffer solution consists of a weak acid and its conjugate base or a. In other words, the amount of acid or base a buffer. This page describes simple acidic and alkaline buffer solutions and explains how they work. Amount of an acid or base that can be added to a volume of a buffer solution before its ph changes. Adding Base To Buffer Solution.
From www.youtube.com
CALCULATION pH OF BUFFER SOLUTION EXAMPLE 2 YouTube Adding Base To Buffer Solution In other words, the amount of acid or base a buffer. A buffer solution is one which resists changes in ph. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize small amounts of added acid or base, thus. What is a buffer solution? This page. Adding Base To Buffer Solution.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint Adding Base To Buffer Solution An aqueous solution that resists any change in ph by adding a small amount of acid or base is called a buffer solution. A buffer solution consists of a weak acid and its conjugate base or a. This page describes simple acidic and alkaline buffer solutions and explains how they work. The buffer is extremely effective at resisting a change. Adding Base To Buffer Solution.
From www.chemistrystudent.com
Buffer Solutions (ALevel) ChemistryStudent Adding Base To Buffer Solution In other words, the amount of acid or base a buffer. A buffer solution consists of a weak acid and its conjugate base or a. The buffer is extremely effective at resisting a change in ph because the added hydroxide ion attacks the weak acid (in very high. Amount of an acid or base that can be added to a. Adding Base To Buffer Solution.
From chemistrytalk.org
What is a Buffer Solution? Chemistry ChemTalk Adding Base To Buffer Solution A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. The buffer solution is a solution able to maintain its hydrogen ion concentration (ph) with only minor changes in the dilution or addition of a. What is a buffer solution? In other words, the amount of acid or base a buffer.. Adding Base To Buffer Solution.
From www.ck12.org
Buffer Solutions Overview ( Video ) Chemistry CK12 Foundation Adding Base To Buffer Solution An aqueous solution that resists any change in ph by adding a small amount of acid or base is called a buffer solution. The buffer is extremely effective at resisting a change in ph because the added hydroxide ion attacks the weak acid (in very high. A buffer is a solution that can resist ph change upon the addition of. Adding Base To Buffer Solution.
From www.geeksforgeeks.org
Buffer Solution Definition, Types, Formula, Examples, and FAQs Adding Base To Buffer Solution What is a buffer solution? The buffer is extremely effective at resisting a change in ph because the added hydroxide ion attacks the weak acid (in very high. An aqueous solution that resists any change in ph by adding a small amount of acid or base is called a buffer solution. This page describes simple acidic and alkaline buffer solutions. Adding Base To Buffer Solution.
From scienceinfo.com
Buffer Solution Types, Properties, and Uses Adding Base To Buffer Solution In other words, the amount of acid or base a buffer. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. The buffer solution is a solution able to maintain its hydrogen ion concentration (ph) with only minor changes in the dilution or addition of a. An aqueous solution that resists. Adding Base To Buffer Solution.
From www.youtube.com
18.2.1 Describe the composition of a buffer solution and explain its Adding Base To Buffer Solution This page describes simple acidic and alkaline buffer solutions and explains how they work. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. What is a buffer solution? A buffer is a solution that can resist ph change upon the addition of. Adding Base To Buffer Solution.