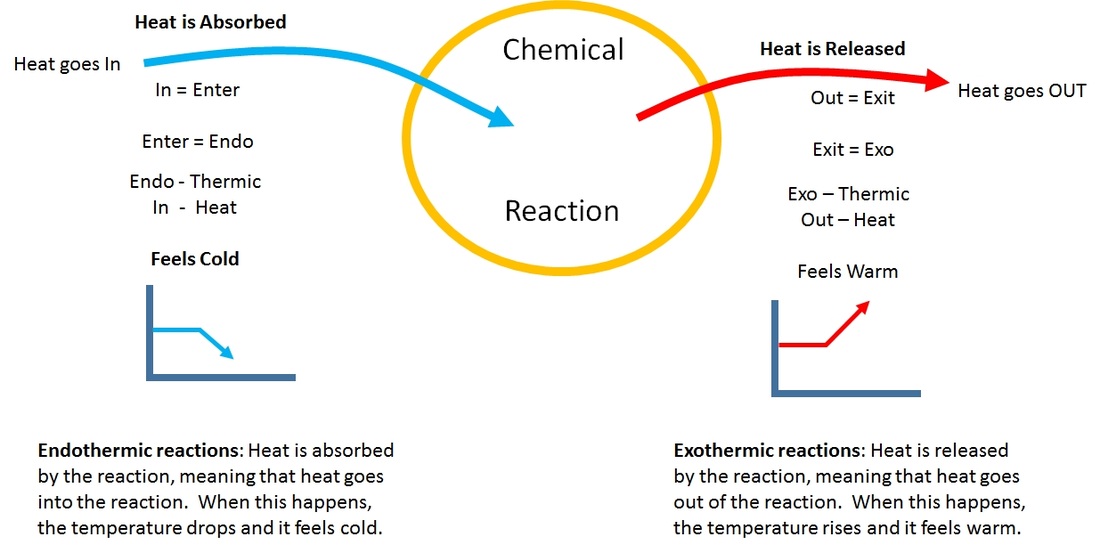
Endothermic Reaction And Exothermic . Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. When a chemical reaction happens, energy is transferred to or from the surroundings. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each which include axis labels, activation energy, and the effect a catalyst would have on the graph. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Describe three ways to speed up a reaction. The categorization of a reaction as endothermic or exothermic depends on the net heat transfer. Combustion is an example of an exothermic reaction. Photosynthesis is a good example of an endothermic reaction. When energy is transferred to the surroundings, this is. Because heat is absorbed, endothermic reactions feel cold. When a chemical reaction occurs, energy is transferred to or from the surroundings. An endothermic reaction occurs when energy is absorbed from the surroundings in the. What's the difference between endothermic and exothermic? Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively.
from
Photosynthesis is a good example of an endothermic reaction. When a chemical reaction occurs, energy is transferred to or from the surroundings. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each which include axis labels, activation energy, and the effect a catalyst would have on the graph. When energy is transferred to the surroundings, this is. The categorization of a reaction as endothermic or exothermic depends on the net heat transfer. What's the difference between endothermic and exothermic? An endothermic reaction occurs when energy is absorbed from the surroundings in the. Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. Describe three ways to speed up a reaction.
Endothermic Reaction And Exothermic Combustion is an example of an exothermic reaction. Combustion is an example of an exothermic reaction. When a chemical reaction happens, energy is transferred to or from the surroundings. Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each which include axis labels, activation energy, and the effect a catalyst would have on the graph. When a chemical reaction occurs, energy is transferred to or from the surroundings. An endothermic reaction occurs when energy is absorbed from the surroundings in the. Describe three ways to speed up a reaction. Photosynthesis is a good example of an endothermic reaction. The categorization of a reaction as endothermic or exothermic depends on the net heat transfer. What's the difference between endothermic and exothermic? Because heat is absorbed, endothermic reactions feel cold. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. When energy is transferred to the surroundings, this is.
From
Endothermic Reaction And Exothermic When energy is transferred to the surroundings, this is. Describe three ways to speed up a reaction. The categorization of a reaction as endothermic or exothermic depends on the net heat transfer. What's the difference between endothermic and exothermic? When a chemical reaction happens, energy is transferred to or from the surroundings. Combustion is an example of an exothermic reaction.. Endothermic Reaction And Exothermic.
From
Endothermic Reaction And Exothermic Photosynthesis is a good example of an endothermic reaction. When energy is transferred to the surroundings, this is. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. When a chemical reaction occurs, energy is transferred to or from the surroundings. The categorization of a reaction as endothermic or exothermic depends on the net heat transfer. An. Endothermic Reaction And Exothermic.
From
Endothermic Reaction And Exothermic Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. Because heat is absorbed, endothermic reactions feel cold. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each which include axis labels, activation energy, and the effect a catalyst would have on the. Endothermic Reaction And Exothermic.
From
Endothermic Reaction And Exothermic When energy is transferred to the surroundings, this is. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each which include axis labels, activation energy, and the effect a catalyst would have on the graph. When a chemical reaction occurs, energy. Endothermic Reaction And Exothermic.
From
Endothermic Reaction And Exothermic Photosynthesis is a good example of an endothermic reaction. When a chemical reaction occurs, energy is transferred to or from the surroundings. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. Describe three ways to speed up a reaction. Because heat is absorbed, endothermic reactions feel cold. Endothermic and exothermic reactions can be thought of as. Endothermic Reaction And Exothermic.
From
Endothermic Reaction And Exothermic An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. The categorization of a reaction as endothermic or exothermic depends on the net heat transfer. Describe three ways to speed up a reaction. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each which include axis labels, activation energy, and. Endothermic Reaction And Exothermic.
From
Endothermic Reaction And Exothermic When a chemical reaction happens, energy is transferred to or from the surroundings. When energy is transferred to the surroundings, this is. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each which include axis labels, activation energy, and the effect a catalyst would have on the graph. Describe three ways to speed up. Endothermic Reaction And Exothermic.
From
Endothermic Reaction And Exothermic Because heat is absorbed, endothermic reactions feel cold. The categorization of a reaction as endothermic or exothermic depends on the net heat transfer. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each which include axis labels, activation energy, and the effect a catalyst would have on the graph. Describe three ways to speed. Endothermic Reaction And Exothermic.
From www.youtube.com
Endothermic Vs. Exothermic Reaction Graphs YouTube Endothermic Reaction And Exothermic An endothermic reaction occurs when energy is absorbed from the surroundings in the. Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each which include axis labels, activation energy, and the effect a. Endothermic Reaction And Exothermic.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Reaction And Exothermic When a chemical reaction occurs, energy is transferred to or from the surroundings. The categorization of a reaction as endothermic or exothermic depends on the net heat transfer. Photosynthesis is a good example of an endothermic reaction. When a chemical reaction happens, energy is transferred to or from the surroundings. An endothermic reaction is a chemical reaction that absorbs thermal. Endothermic Reaction And Exothermic.
From
Endothermic Reaction And Exothermic When energy is transferred to the surroundings, this is. An endothermic reaction occurs when energy is absorbed from the surroundings in the. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each which include axis labels, activation energy, and the effect a catalyst would have on the graph. When a chemical reaction happens, energy. Endothermic Reaction And Exothermic.
From
Endothermic Reaction And Exothermic Photosynthesis is a good example of an endothermic reaction. When energy is transferred to the surroundings, this is. What's the difference between endothermic and exothermic? An endothermic reaction occurs when energy is absorbed from the surroundings in the. Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. Because. Endothermic Reaction And Exothermic.
From www.slideserve.com
PPT Endothermic Vs. Exothermic Reaction Graphs PowerPoint Endothermic Reaction And Exothermic Combustion is an example of an exothermic reaction. Describe three ways to speed up a reaction. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each which include axis labels, activation energy, and the effect a catalyst would have on the graph. Because heat is absorbed, endothermic reactions feel cold. When a chemical reaction. Endothermic Reaction And Exothermic.
From
Endothermic Reaction And Exothermic When a chemical reaction happens, energy is transferred to or from the surroundings. Photosynthesis is a good example of an endothermic reaction. Because heat is absorbed, endothermic reactions feel cold. When energy is transferred to the surroundings, this is. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each which include axis labels, activation. Endothermic Reaction And Exothermic.
From
Endothermic Reaction And Exothermic When a chemical reaction happens, energy is transferred to or from the surroundings. Because heat is absorbed, endothermic reactions feel cold. Combustion is an example of an exothermic reaction. When energy is transferred to the surroundings, this is. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. When a chemical reaction occurs, energy is transferred. Endothermic Reaction And Exothermic.
From
Endothermic Reaction And Exothermic Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. Combustion is an example of an exothermic reaction. When a chemical reaction occurs, energy is transferred to or from the surroundings. Photosynthesis is a good example of an endothermic reaction. Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the. Endothermic Reaction And Exothermic.
From question.pandai.org
Endothermic and exothermic reactions Endothermic Reaction And Exothermic Combustion is an example of an exothermic reaction. When a chemical reaction occurs, energy is transferred to or from the surroundings. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each which include axis labels, activation energy, and the effect a catalyst would have on the graph. Photosynthesis is a good example of an. Endothermic Reaction And Exothermic.
From
Endothermic Reaction And Exothermic The categorization of a reaction as endothermic or exothermic depends on the net heat transfer. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Combustion is an example of an exothermic reaction. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each which include axis labels, activation energy, and. Endothermic Reaction And Exothermic.
From revisechemistry.uk
Exothermic and Endothermic Reactions AQA C5 revisechemistry.uk Endothermic Reaction And Exothermic Photosynthesis is a good example of an endothermic reaction. Describe three ways to speed up a reaction. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each which include axis labels, activation energy, and the effect a catalyst would have on the graph. Endothermic and exothermic reactions can be thought of as having energy. Endothermic Reaction And Exothermic.
From stewart-switch.com
Exothermic And Endothermic Reaction Diagram Endothermic Reaction And Exothermic Photosynthesis is a good example of an endothermic reaction. Describe three ways to speed up a reaction. When energy is transferred to the surroundings, this is. The categorization of a reaction as endothermic or exothermic depends on the net heat transfer. When a chemical reaction happens, energy is transferred to or from the surroundings. Endothermic and exothermic reactions can be. Endothermic Reaction And Exothermic.
From storyguest.blogspot.com
A Chemical Equation Tells The Story Of A Chemical Reaction Story Guest Endothermic Reaction And Exothermic Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each which include axis labels, activation energy, and the effect a catalyst would have on the graph. The categorization of a reaction as endothermic or exothermic depends on the net heat transfer. When a chemical reaction happens, energy is transferred to or from the surroundings.. Endothermic Reaction And Exothermic.
From
Endothermic Reaction And Exothermic What's the difference between endothermic and exothermic? Describe three ways to speed up a reaction. The categorization of a reaction as endothermic or exothermic depends on the net heat transfer. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. Because heat is absorbed, endothermic reactions feel cold. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions. Endothermic Reaction And Exothermic.
From vhmsscience.weebly.com
Endo/Exothermic Reactions VISTA HEIGHTS 8TH GRADE SCIENCE Endothermic Reaction And Exothermic An endothermic reaction occurs when energy is absorbed from the surroundings in the. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each which include axis labels, activation energy, and the effect a catalyst would have on the graph. When a chemical reaction occurs, energy is transferred to or from the surroundings. Endothermic and. Endothermic Reaction And Exothermic.
From askanydifference.com
Exothermic vs Endothermic Reaction Difference and Comparison Endothermic Reaction And Exothermic Combustion is an example of an exothermic reaction. Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. When a chemical reaction happens, energy is transferred to or from the surroundings. The categorization of a reaction as endothermic or exothermic depends on the net heat transfer. Photosynthesis is a. Endothermic Reaction And Exothermic.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Reaction And Exothermic What's the difference between endothermic and exothermic? When a chemical reaction happens, energy is transferred to or from the surroundings. Photosynthesis is a good example of an endothermic reaction. An endothermic reaction occurs when energy is absorbed from the surroundings in the. When a chemical reaction occurs, energy is transferred to or from the surroundings. Combustion is an example of. Endothermic Reaction And Exothermic.
From
Endothermic Reaction And Exothermic Combustion is an example of an exothermic reaction. Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. Photosynthesis is a good example of an endothermic reaction. Describe three ways to speed up a reaction. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings.. Endothermic Reaction And Exothermic.
From
Endothermic Reaction And Exothermic Describe three ways to speed up a reaction. When energy is transferred to the surroundings, this is. Photosynthesis is a good example of an endothermic reaction. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each which include axis labels, activation energy, and the effect a catalyst would have on the graph. Endothermic and. Endothermic Reaction And Exothermic.
From
Endothermic Reaction And Exothermic Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. What's the difference between endothermic and exothermic? An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Because heat is absorbed, endothermic reactions feel cold. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and. Endothermic Reaction And Exothermic.
From
Endothermic Reaction And Exothermic When energy is transferred to the surroundings, this is. When a chemical reaction happens, energy is transferred to or from the surroundings. The categorization of a reaction as endothermic or exothermic depends on the net heat transfer. Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. Photosynthesis is. Endothermic Reaction And Exothermic.
From
Endothermic Reaction And Exothermic When a chemical reaction occurs, energy is transferred to or from the surroundings. An endothermic reaction occurs when energy is absorbed from the surroundings in the. What's the difference between endothermic and exothermic? Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. Photosynthesis is a good example of. Endothermic Reaction And Exothermic.
From
Endothermic Reaction And Exothermic Because heat is absorbed, endothermic reactions feel cold. When a chemical reaction happens, energy is transferred to or from the surroundings. An endothermic reaction occurs when energy is absorbed from the surroundings in the. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Endothermic and exothermic reactions are chemical reactions that absorb and release heat,. Endothermic Reaction And Exothermic.
From
Endothermic Reaction And Exothermic Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. When a chemical reaction occurs, energy is transferred to or from the surroundings. Photosynthesis is a good example of an endothermic reaction. When a chemical reaction happens, energy is transferred to or from the surroundings. What's the difference between. Endothermic Reaction And Exothermic.
From
Endothermic Reaction And Exothermic When energy is transferred to the surroundings, this is. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. The categorization of a reaction as endothermic or exothermic depends on the net heat transfer. Differentiate between. Endothermic Reaction And Exothermic.
From
Endothermic Reaction And Exothermic Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. Photosynthesis is a good example of an endothermic reaction. What's the difference between endothermic and exothermic? Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each which include axis labels, activation energy, and. Endothermic Reaction And Exothermic.
From pediaa.com
Difference Between Endothermic and Exothermic Reactions Definition Endothermic Reaction And Exothermic What's the difference between endothermic and exothermic? Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. Photosynthesis is a good example of an endothermic reaction. An endothermic reaction occurs when energy is absorbed from the surroundings in the. Describe three ways to speed up a reaction. Endothermic and exothermic reactions can be thought of as having. Endothermic Reaction And Exothermic.