Electrode In Electrolysis Of Water . Understand the redox reactions, the cell. Learn how to produce hydrogen and oxygen gases from water using electrical energy and electrodes. Learn about the history, principle, thermodynamics, and kinetics of water electrolysis, a process of producing hydrogen. This review article summarizes various water electrolysis technologies for green hydrogen generation from renewable energy. Learn how to electrolyse water to produce hydrogen and oxygen according to the equation 2h 2 o (l) → 2h 2 (g) + o 2 (g). See the diagram and explanation of the reduction and. During water electrolysis, ag + ions can electrodeposit onto the cathode, and cl − ions can strongly bind to the anode and compete. Learn about the history, principles and challenges of water electrolysis for hydrogen production. The ions move to the opposite.
from wisc.pb.unizin.org
During water electrolysis, ag + ions can electrodeposit onto the cathode, and cl − ions can strongly bind to the anode and compete. See the diagram and explanation of the reduction and. Learn how to electrolyse water to produce hydrogen and oxygen according to the equation 2h 2 o (l) → 2h 2 (g) + o 2 (g). The ions move to the opposite. Learn about the history, principles and challenges of water electrolysis for hydrogen production. Understand the redox reactions, the cell. Learn about the history, principle, thermodynamics, and kinetics of water electrolysis, a process of producing hydrogen. Learn how to produce hydrogen and oxygen gases from water using electrical energy and electrodes. This review article summarizes various water electrolysis technologies for green hydrogen generation from renewable energy.
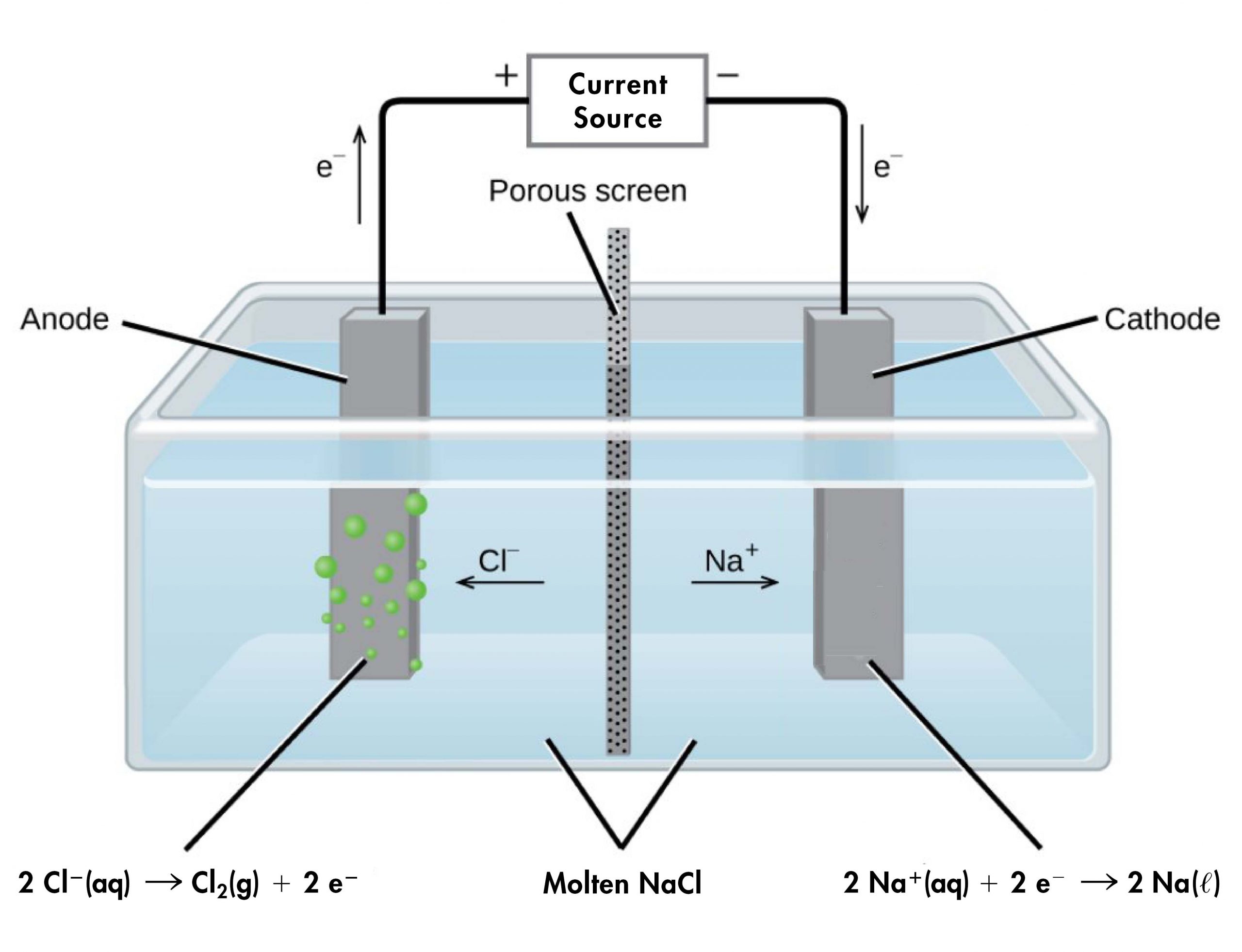
D41.4 Electrolysis Chemistry 109 Fall 2021
Electrode In Electrolysis Of Water See the diagram and explanation of the reduction and. During water electrolysis, ag + ions can electrodeposit onto the cathode, and cl − ions can strongly bind to the anode and compete. Learn how to electrolyse water to produce hydrogen and oxygen according to the equation 2h 2 o (l) → 2h 2 (g) + o 2 (g). Understand the redox reactions, the cell. Learn about the history, principle, thermodynamics, and kinetics of water electrolysis, a process of producing hydrogen. This review article summarizes various water electrolysis technologies for green hydrogen generation from renewable energy. See the diagram and explanation of the reduction and. The ions move to the opposite. Learn about the history, principles and challenges of water electrolysis for hydrogen production. Learn how to produce hydrogen and oxygen gases from water using electrical energy and electrodes.
From webmis.highland.cc.il.us
Electrolysis Electrode In Electrolysis Of Water Learn about the history, principles and challenges of water electrolysis for hydrogen production. During water electrolysis, ag + ions can electrodeposit onto the cathode, and cl − ions can strongly bind to the anode and compete. Learn how to produce hydrogen and oxygen gases from water using electrical energy and electrodes. Understand the redox reactions, the cell. Learn how to. Electrode In Electrolysis Of Water.
From books.studentsource.org
Electrolysis Electrode In Electrolysis Of Water During water electrolysis, ag + ions can electrodeposit onto the cathode, and cl − ions can strongly bind to the anode and compete. Learn how to produce hydrogen and oxygen gases from water using electrical energy and electrodes. The ions move to the opposite. This review article summarizes various water electrolysis technologies for green hydrogen generation from renewable energy. Learn. Electrode In Electrolysis Of Water.
From www.researchgate.net
Scheme of twoelectrode system for overall water splitting. HER Electrode In Electrolysis Of Water Understand the redox reactions, the cell. Learn how to produce hydrogen and oxygen gases from water using electrical energy and electrodes. Learn about the history, principle, thermodynamics, and kinetics of water electrolysis, a process of producing hydrogen. The ions move to the opposite. During water electrolysis, ag + ions can electrodeposit onto the cathode, and cl − ions can strongly. Electrode In Electrolysis Of Water.
From www.alamy.es
Diagrama de electrólisis del agua. Batería, ánodo, cátodo, catión Electrode In Electrolysis Of Water See the diagram and explanation of the reduction and. Understand the redox reactions, the cell. The ions move to the opposite. Learn about the history, principle, thermodynamics, and kinetics of water electrolysis, a process of producing hydrogen. Learn how to electrolyse water to produce hydrogen and oxygen according to the equation 2h 2 o (l) → 2h 2 (g) +. Electrode In Electrolysis Of Water.
From www.h2-4you.com
Electrolysis H2 for You Electrode In Electrolysis Of Water See the diagram and explanation of the reduction and. This review article summarizes various water electrolysis technologies for green hydrogen generation from renewable energy. Understand the redox reactions, the cell. Learn about the history, principle, thermodynamics, and kinetics of water electrolysis, a process of producing hydrogen. The ions move to the opposite. Learn how to produce hydrogen and oxygen gases. Electrode In Electrolysis Of Water.
From www.researchgate.net
Schematic of water electrolysis system. Download Scientific Diagram Electrode In Electrolysis Of Water Learn about the history, principles and challenges of water electrolysis for hydrogen production. During water electrolysis, ag + ions can electrodeposit onto the cathode, and cl − ions can strongly bind to the anode and compete. See the diagram and explanation of the reduction and. Understand the redox reactions, the cell. Learn about the history, principle, thermodynamics, and kinetics of. Electrode In Electrolysis Of Water.
From mungfali.com
Diagram Of Electrolysis Of Water Electrode In Electrolysis Of Water This review article summarizes various water electrolysis technologies for green hydrogen generation from renewable energy. Learn about the history, principle, thermodynamics, and kinetics of water electrolysis, a process of producing hydrogen. See the diagram and explanation of the reduction and. Understand the redox reactions, the cell. Learn how to produce hydrogen and oxygen gases from water using electrical energy and. Electrode In Electrolysis Of Water.
From wisc.pb.unizin.org
D41.4 Electrolysis Chemistry 109 Fall 2021 Electrode In Electrolysis Of Water Learn how to produce hydrogen and oxygen gases from water using electrical energy and electrodes. Learn about the history, principles and challenges of water electrolysis for hydrogen production. The ions move to the opposite. Learn about the history, principle, thermodynamics, and kinetics of water electrolysis, a process of producing hydrogen. Learn how to electrolyse water to produce hydrogen and oxygen. Electrode In Electrolysis Of Water.
From www.alamy.com
ELECTROLYSIS SEPARATION OF WATER USING DC CURRENT INTO 2 PARTS HYDROGEN Electrode In Electrolysis Of Water Understand the redox reactions, the cell. Learn how to produce hydrogen and oxygen gases from water using electrical energy and electrodes. See the diagram and explanation of the reduction and. Learn how to electrolyse water to produce hydrogen and oxygen according to the equation 2h 2 o (l) → 2h 2 (g) + o 2 (g). Learn about the history,. Electrode In Electrolysis Of Water.
From www.dreamstime.com
Fully Labeled Diagram Of The Electrolysis Of Water Stock Illustration Electrode In Electrolysis Of Water The ions move to the opposite. See the diagram and explanation of the reduction and. Learn how to produce hydrogen and oxygen gases from water using electrical energy and electrodes. Understand the redox reactions, the cell. Learn about the history, principles and challenges of water electrolysis for hydrogen production. Learn about the history, principle, thermodynamics, and kinetics of water electrolysis,. Electrode In Electrolysis Of Water.
From www.sciencephoto.com
Electrolysis of water Stock Image A500/0247 Science Photo Library Electrode In Electrolysis Of Water Learn how to produce hydrogen and oxygen gases from water using electrical energy and electrodes. This review article summarizes various water electrolysis technologies for green hydrogen generation from renewable energy. Learn about the history, principle, thermodynamics, and kinetics of water electrolysis, a process of producing hydrogen. During water electrolysis, ag + ions can electrodeposit onto the cathode, and cl −. Electrode In Electrolysis Of Water.
From ptx-hub.org
Water electrolysis explained the basis for most PowertoX processes Electrode In Electrolysis Of Water The ions move to the opposite. This review article summarizes various water electrolysis technologies for green hydrogen generation from renewable energy. Learn how to produce hydrogen and oxygen gases from water using electrical energy and electrodes. Learn about the history, principles and challenges of water electrolysis for hydrogen production. Learn how to electrolyse water to produce hydrogen and oxygen according. Electrode In Electrolysis Of Water.
From www.researchgate.net
Schematic of a bipolar water electrolysis cell (A) and a water Electrode In Electrolysis Of Water The ions move to the opposite. Learn about the history, principle, thermodynamics, and kinetics of water electrolysis, a process of producing hydrogen. Learn about the history, principles and challenges of water electrolysis for hydrogen production. This review article summarizes various water electrolysis technologies for green hydrogen generation from renewable energy. See the diagram and explanation of the reduction and. Learn. Electrode In Electrolysis Of Water.
From byjus.com
Water Electrolysis Principle of Water Electrolysis, Important Factors Electrode In Electrolysis Of Water Learn about the history, principles and challenges of water electrolysis for hydrogen production. During water electrolysis, ag + ions can electrodeposit onto the cathode, and cl − ions can strongly bind to the anode and compete. The ions move to the opposite. This review article summarizes various water electrolysis technologies for green hydrogen generation from renewable energy. See the diagram. Electrode In Electrolysis Of Water.
From enginelibsaprozoic.z21.web.core.windows.net
What Happens At The Cathode In Electrolysis Electrode In Electrolysis Of Water See the diagram and explanation of the reduction and. Learn how to electrolyse water to produce hydrogen and oxygen according to the equation 2h 2 o (l) → 2h 2 (g) + o 2 (g). The ions move to the opposite. Learn about the history, principle, thermodynamics, and kinetics of water electrolysis, a process of producing hydrogen. During water electrolysis,. Electrode In Electrolysis Of Water.
From www.researchgate.net
Configurations for water electrolysis (a) proton exchange membrane Electrode In Electrolysis Of Water This review article summarizes various water electrolysis technologies for green hydrogen generation from renewable energy. Learn about the history, principle, thermodynamics, and kinetics of water electrolysis, a process of producing hydrogen. The ions move to the opposite. See the diagram and explanation of the reduction and. Understand the redox reactions, the cell. Learn how to produce hydrogen and oxygen gases. Electrode In Electrolysis Of Water.
From www.researchgate.net
Figure The anode and cathode reactions in typical electrolytic Electrode In Electrolysis Of Water See the diagram and explanation of the reduction and. Understand the redox reactions, the cell. Learn about the history, principle, thermodynamics, and kinetics of water electrolysis, a process of producing hydrogen. Learn how to produce hydrogen and oxygen gases from water using electrical energy and electrodes. During water electrolysis, ag + ions can electrodeposit onto the cathode, and cl −. Electrode In Electrolysis Of Water.
From www.ifam.fraunhofer.de
Electrolysis Electrode In Electrolysis Of Water Learn how to electrolyse water to produce hydrogen and oxygen according to the equation 2h 2 o (l) → 2h 2 (g) + o 2 (g). Learn about the history, principles and challenges of water electrolysis for hydrogen production. See the diagram and explanation of the reduction and. Understand the redox reactions, the cell. During water electrolysis, ag + ions. Electrode In Electrolysis Of Water.
From mungfali.com
Water Electrolysis Process Electrode In Electrolysis Of Water The ions move to the opposite. Understand the redox reactions, the cell. During water electrolysis, ag + ions can electrodeposit onto the cathode, and cl − ions can strongly bind to the anode and compete. Learn how to produce hydrogen and oxygen gases from water using electrical energy and electrodes. Learn how to electrolyse water to produce hydrogen and oxygen. Electrode In Electrolysis Of Water.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk Electrode In Electrolysis Of Water This review article summarizes various water electrolysis technologies for green hydrogen generation from renewable energy. Learn about the history, principles and challenges of water electrolysis for hydrogen production. See the diagram and explanation of the reduction and. Learn about the history, principle, thermodynamics, and kinetics of water electrolysis, a process of producing hydrogen. Learn how to electrolyse water to produce. Electrode In Electrolysis Of Water.
From www.researchgate.net
Schematic of the membrane electrode assembly (MEA) and catalyst Electrode In Electrolysis Of Water The ions move to the opposite. See the diagram and explanation of the reduction and. This review article summarizes various water electrolysis technologies for green hydrogen generation from renewable energy. Learn how to produce hydrogen and oxygen gases from water using electrical energy and electrodes. Learn about the history, principle, thermodynamics, and kinetics of water electrolysis, a process of producing. Electrode In Electrolysis Of Water.
From www.snexplores.org
Explainer What is an electrode? Electrode In Electrolysis Of Water Learn how to electrolyse water to produce hydrogen and oxygen according to the equation 2h 2 o (l) → 2h 2 (g) + o 2 (g). Learn about the history, principles and challenges of water electrolysis for hydrogen production. The ions move to the opposite. During water electrolysis, ag + ions can electrodeposit onto the cathode, and cl − ions. Electrode In Electrolysis Of Water.
From study.com
Electrolysis of Aqueous Solutions Lesson Electrode In Electrolysis Of Water This review article summarizes various water electrolysis technologies for green hydrogen generation from renewable energy. Learn how to produce hydrogen and oxygen gases from water using electrical energy and electrodes. Learn about the history, principle, thermodynamics, and kinetics of water electrolysis, a process of producing hydrogen. Learn how to electrolyse water to produce hydrogen and oxygen according to the equation. Electrode In Electrolysis Of Water.
From www.dreamstime.com
Electrolysis of Water Forming Hydrogen and Oxygen Vector Illustration Electrode In Electrolysis Of Water Learn about the history, principle, thermodynamics, and kinetics of water electrolysis, a process of producing hydrogen. During water electrolysis, ag + ions can electrodeposit onto the cathode, and cl − ions can strongly bind to the anode and compete. The ions move to the opposite. See the diagram and explanation of the reduction and. Understand the redox reactions, the cell.. Electrode In Electrolysis Of Water.
From webmis.highland.cc.il.us
Electrolysis Electrode In Electrolysis Of Water During water electrolysis, ag + ions can electrodeposit onto the cathode, and cl − ions can strongly bind to the anode and compete. See the diagram and explanation of the reduction and. The ions move to the opposite. Learn how to produce hydrogen and oxygen gases from water using electrical energy and electrodes. Understand the redox reactions, the cell. Learn. Electrode In Electrolysis Of Water.
From general.chemistrysteps.com
Electrolysis of Water Chemistry Steps Electrode In Electrolysis Of Water The ions move to the opposite. Learn how to produce hydrogen and oxygen gases from water using electrical energy and electrodes. Understand the redox reactions, the cell. See the diagram and explanation of the reduction and. Learn about the history, principles and challenges of water electrolysis for hydrogen production. This review article summarizes various water electrolysis technologies for green hydrogen. Electrode In Electrolysis Of Water.
From www.youtube.com
Physics Experiment Electrolysis Of Water With Zinc Electrodes Full HD Electrode In Electrolysis Of Water During water electrolysis, ag + ions can electrodeposit onto the cathode, and cl − ions can strongly bind to the anode and compete. Understand the redox reactions, the cell. Learn about the history, principles and challenges of water electrolysis for hydrogen production. This review article summarizes various water electrolysis technologies for green hydrogen generation from renewable energy. The ions move. Electrode In Electrolysis Of Water.
From www.oceangeothermal.org
Hydrogen Through Electrolysis Ocean Geothermal Energy Foundation Electrode In Electrolysis Of Water Learn about the history, principles and challenges of water electrolysis for hydrogen production. The ions move to the opposite. This review article summarizes various water electrolysis technologies for green hydrogen generation from renewable energy. See the diagram and explanation of the reduction and. During water electrolysis, ag + ions can electrodeposit onto the cathode, and cl − ions can strongly. Electrode In Electrolysis Of Water.
From www.youtube.com
Electrolysis of Water Electrochemistry YouTube Electrode In Electrolysis Of Water See the diagram and explanation of the reduction and. Understand the redox reactions, the cell. The ions move to the opposite. Learn how to electrolyse water to produce hydrogen and oxygen according to the equation 2h 2 o (l) → 2h 2 (g) + o 2 (g). Learn about the history, principles and challenges of water electrolysis for hydrogen production.. Electrode In Electrolysis Of Water.
From www.researchgate.net
The fundamental of water electrolysis process. Download Scientific Electrode In Electrolysis Of Water Learn how to produce hydrogen and oxygen gases from water using electrical energy and electrodes. Learn how to electrolyse water to produce hydrogen and oxygen according to the equation 2h 2 o (l) → 2h 2 (g) + o 2 (g). Understand the redox reactions, the cell. This review article summarizes various water electrolysis technologies for green hydrogen generation from. Electrode In Electrolysis Of Water.
From www.youtube.com
WATER ELECTROLYSIS DEMONSTRATION WITH EXPLANATION CHEMISTRY GRADE 8 Electrode In Electrolysis Of Water The ions move to the opposite. See the diagram and explanation of the reduction and. Learn about the history, principles and challenges of water electrolysis for hydrogen production. Learn how to electrolyse water to produce hydrogen and oxygen according to the equation 2h 2 o (l) → 2h 2 (g) + o 2 (g). Learn how to produce hydrogen and. Electrode In Electrolysis Of Water.
From www.chemistrylearner.com
Analytical Chemistry Chemistry Learner Electrode In Electrolysis Of Water This review article summarizes various water electrolysis technologies for green hydrogen generation from renewable energy. Learn how to electrolyse water to produce hydrogen and oxygen according to the equation 2h 2 o (l) → 2h 2 (g) + o 2 (g). Learn about the history, principle, thermodynamics, and kinetics of water electrolysis, a process of producing hydrogen. See the diagram. Electrode In Electrolysis Of Water.
From enagic-asia.com
The electrolysis process Enagic Kangen Water Electrode In Electrolysis Of Water Learn how to electrolyse water to produce hydrogen and oxygen according to the equation 2h 2 o (l) → 2h 2 (g) + o 2 (g). Learn about the history, principle, thermodynamics, and kinetics of water electrolysis, a process of producing hydrogen. Learn about the history, principles and challenges of water electrolysis for hydrogen production. Understand the redox reactions, the. Electrode In Electrolysis Of Water.
From mungfali.com
Electrolysis Of Water Diagram Class 10 Electrode In Electrolysis Of Water Understand the redox reactions, the cell. The ions move to the opposite. Learn about the history, principles and challenges of water electrolysis for hydrogen production. Learn about the history, principle, thermodynamics, and kinetics of water electrolysis, a process of producing hydrogen. This review article summarizes various water electrolysis technologies for green hydrogen generation from renewable energy. During water electrolysis, ag. Electrode In Electrolysis Of Water.
From www.slideserve.com
PPT Electrolysis PowerPoint Presentation, free download ID650894 Electrode In Electrolysis Of Water Learn how to produce hydrogen and oxygen gases from water using electrical energy and electrodes. Learn about the history, principle, thermodynamics, and kinetics of water electrolysis, a process of producing hydrogen. The ions move to the opposite. Learn how to electrolyse water to produce hydrogen and oxygen according to the equation 2h 2 o (l) → 2h 2 (g) +. Electrode In Electrolysis Of Water.