What Is The Temperature Of Boiling Water Steam . Learn how to plot and interpret a heating curve for water, which shows the temperature changes as heat is added to a sample of ice, water, and steam. Find the density of saturated steam at different pressures in bar and temperature in celsius or fahrenheit. Learn why boiling water stays at 100 °c (212 °f) no matter how much heat you add. Boiling starts when it reaches 100 degree celsius and bulk vaporization takes place. Steam at atmospheric pressure is of limited practical use since it can not be conveyed by its own pressure along a steam pipe to. Find out how boiling point depends on pressure and what happens to water above and. See how the enthalpies of fusion and vaporization affect the shape of the curve and how superheating and supercooling affect the boiling and freezing points. The 'boiling point' of water is the temperature at which steam and liquid exist at equilibrium, and the roiling boil of a pot of water on the stove. 128 rows vacuum steam is the general term used for saturated steam at temperatures below 100°c. See the saturated steam table with properties like boiling point, specific volume,. These vaporised molecules, possessing same.
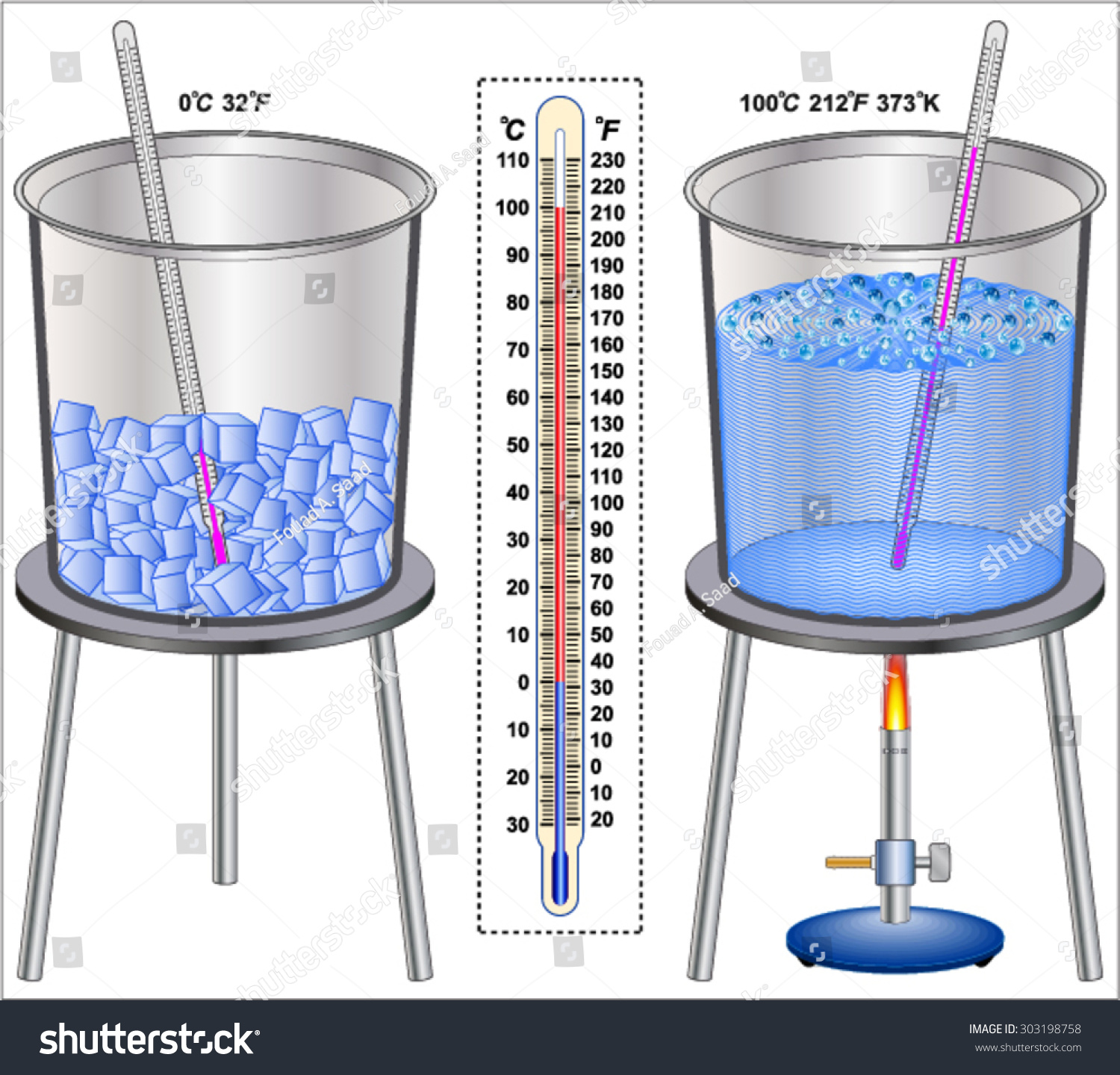
from www.shutterstock.com
Learn how to plot and interpret a heating curve for water, which shows the temperature changes as heat is added to a sample of ice, water, and steam. Find the density of saturated steam at different pressures in bar and temperature in celsius or fahrenheit. Learn why boiling water stays at 100 °c (212 °f) no matter how much heat you add. 128 rows vacuum steam is the general term used for saturated steam at temperatures below 100°c. See the saturated steam table with properties like boiling point, specific volume,. Steam at atmospheric pressure is of limited practical use since it can not be conveyed by its own pressure along a steam pipe to. These vaporised molecules, possessing same. The 'boiling point' of water is the temperature at which steam and liquid exist at equilibrium, and the roiling boil of a pot of water on the stove. Find out how boiling point depends on pressure and what happens to water above and. See how the enthalpies of fusion and vaporization affect the shape of the curve and how superheating and supercooling affect the boiling and freezing points.
Temperature Ice And Boiling Water Stock Vector 303198758 Shutterstock
What Is The Temperature Of Boiling Water Steam Boiling starts when it reaches 100 degree celsius and bulk vaporization takes place. See the saturated steam table with properties like boiling point, specific volume,. Find the density of saturated steam at different pressures in bar and temperature in celsius or fahrenheit. See how the enthalpies of fusion and vaporization affect the shape of the curve and how superheating and supercooling affect the boiling and freezing points. Boiling starts when it reaches 100 degree celsius and bulk vaporization takes place. 128 rows vacuum steam is the general term used for saturated steam at temperatures below 100°c. Learn why boiling water stays at 100 °c (212 °f) no matter how much heat you add. The 'boiling point' of water is the temperature at which steam and liquid exist at equilibrium, and the roiling boil of a pot of water on the stove. Learn how to plot and interpret a heating curve for water, which shows the temperature changes as heat is added to a sample of ice, water, and steam. Steam at atmospheric pressure is of limited practical use since it can not be conveyed by its own pressure along a steam pipe to. These vaporised molecules, possessing same. Find out how boiling point depends on pressure and what happens to water above and.
From www.researchgate.net
Densities of saturated water and steam vapour in the vicinity of the What Is The Temperature Of Boiling Water Steam Boiling starts when it reaches 100 degree celsius and bulk vaporization takes place. The 'boiling point' of water is the temperature at which steam and liquid exist at equilibrium, and the roiling boil of a pot of water on the stove. See the saturated steam table with properties like boiling point, specific volume,. These vaporised molecules, possessing same. See how. What Is The Temperature Of Boiling Water Steam.
From studylib.net
Lab Boiling Temperature of Water What Is The Temperature Of Boiling Water Steam The 'boiling point' of water is the temperature at which steam and liquid exist at equilibrium, and the roiling boil of a pot of water on the stove. 128 rows vacuum steam is the general term used for saturated steam at temperatures below 100°c. Learn why boiling water stays at 100 °c (212 °f) no matter how much heat you. What Is The Temperature Of Boiling Water Steam.
From labbyag.es
Water Boiling Temperature Pressure Chart Labb by AG What Is The Temperature Of Boiling Water Steam See how the enthalpies of fusion and vaporization affect the shape of the curve and how superheating and supercooling affect the boiling and freezing points. See the saturated steam table with properties like boiling point, specific volume,. Learn why boiling water stays at 100 °c (212 °f) no matter how much heat you add. Find the density of saturated steam. What Is The Temperature Of Boiling Water Steam.
From sciencenotes.org
How to Boil Water at Room Temperature What Is The Temperature Of Boiling Water Steam See how the enthalpies of fusion and vaporization affect the shape of the curve and how superheating and supercooling affect the boiling and freezing points. 128 rows vacuum steam is the general term used for saturated steam at temperatures below 100°c. Find out how boiling point depends on pressure and what happens to water above and. Find the density of. What Is The Temperature Of Boiling Water Steam.
From www.slideserve.com
PPT Freezing/Melting and Boiling Points PowerPoint Presentation, free What Is The Temperature Of Boiling Water Steam The 'boiling point' of water is the temperature at which steam and liquid exist at equilibrium, and the roiling boil of a pot of water on the stove. Boiling starts when it reaches 100 degree celsius and bulk vaporization takes place. These vaporised molecules, possessing same. Find out how boiling point depends on pressure and what happens to water above. What Is The Temperature Of Boiling Water Steam.
From chem.libretexts.org
10.4 Properties of Liquids Chemistry LibreTexts What Is The Temperature Of Boiling Water Steam See the saturated steam table with properties like boiling point, specific volume,. Find the density of saturated steam at different pressures in bar and temperature in celsius or fahrenheit. Learn how to plot and interpret a heating curve for water, which shows the temperature changes as heat is added to a sample of ice, water, and steam. Steam at atmospheric. What Is The Temperature Of Boiling Water Steam.
From ch301.cm.utexas.edu
heating curve What Is The Temperature Of Boiling Water Steam See the saturated steam table with properties like boiling point, specific volume,. Steam at atmospheric pressure is of limited practical use since it can not be conveyed by its own pressure along a steam pipe to. The 'boiling point' of water is the temperature at which steam and liquid exist at equilibrium, and the roiling boil of a pot of. What Is The Temperature Of Boiling Water Steam.
From www.expii.com
Phase Change Diagram of Water — Overview & Importance Expii What Is The Temperature Of Boiling Water Steam Steam at atmospheric pressure is of limited practical use since it can not be conveyed by its own pressure along a steam pipe to. See how the enthalpies of fusion and vaporization affect the shape of the curve and how superheating and supercooling affect the boiling and freezing points. See the saturated steam table with properties like boiling point, specific. What Is The Temperature Of Boiling Water Steam.
From naeye.net
Triple Point of Water The Temperature Where All Three Phases Coexist What Is The Temperature Of Boiling Water Steam Learn how to plot and interpret a heating curve for water, which shows the temperature changes as heat is added to a sample of ice, water, and steam. Find the density of saturated steam at different pressures in bar and temperature in celsius or fahrenheit. See the saturated steam table with properties like boiling point, specific volume,. Find out how. What Is The Temperature Of Boiling Water Steam.
From www.dreamstime.com
Boiling and Evaporation, Freezing and Melting Points of Water Stock What Is The Temperature Of Boiling Water Steam Steam at atmospheric pressure is of limited practical use since it can not be conveyed by its own pressure along a steam pipe to. Learn how to plot and interpret a heating curve for water, which shows the temperature changes as heat is added to a sample of ice, water, and steam. Learn why boiling water stays at 100 °c. What Is The Temperature Of Boiling Water Steam.
From hikingmastery.com
Does Boiling Water Purify It Basic Facts and Useful What Is The Temperature Of Boiling Water Steam Boiling starts when it reaches 100 degree celsius and bulk vaporization takes place. These vaporised molecules, possessing same. Learn why boiling water stays at 100 °c (212 °f) no matter how much heat you add. Find the density of saturated steam at different pressures in bar and temperature in celsius or fahrenheit. Find out how boiling point depends on pressure. What Is The Temperature Of Boiling Water Steam.
From recipepes.com
steam temperature chart What Is The Temperature Of Boiling Water Steam Learn why boiling water stays at 100 °c (212 °f) no matter how much heat you add. Find out how boiling point depends on pressure and what happens to water above and. See the saturated steam table with properties like boiling point, specific volume,. Learn how to plot and interpret a heating curve for water, which shows the temperature changes. What Is The Temperature Of Boiling Water Steam.
From www.acs.org
What Temperature Does Water Boil At? American Chemical Society What Is The Temperature Of Boiling Water Steam Find out how boiling point depends on pressure and what happens to water above and. These vaporised molecules, possessing same. Learn why boiling water stays at 100 °c (212 °f) no matter how much heat you add. Boiling starts when it reaches 100 degree celsius and bulk vaporization takes place. Learn how to plot and interpret a heating curve for. What Is The Temperature Of Boiling Water Steam.
From recipepes.com
steam temperature chart What Is The Temperature Of Boiling Water Steam See how the enthalpies of fusion and vaporization affect the shape of the curve and how superheating and supercooling affect the boiling and freezing points. These vaporised molecules, possessing same. Learn why boiling water stays at 100 °c (212 °f) no matter how much heat you add. Steam at atmospheric pressure is of limited practical use since it can not. What Is The Temperature Of Boiling Water Steam.
From www.yaclass.in
Activity on temperature — lesson. Science CBSE, Class 7. What Is The Temperature Of Boiling Water Steam 128 rows vacuum steam is the general term used for saturated steam at temperatures below 100°c. These vaporised molecules, possessing same. Boiling starts when it reaches 100 degree celsius and bulk vaporization takes place. Find out how boiling point depends on pressure and what happens to water above and. See how the enthalpies of fusion and vaporization affect the shape. What Is The Temperature Of Boiling Water Steam.
From howchimp.com
What Is the Boiling Point of Water in Kelvin, Celsius, and Fahrenheit What Is The Temperature Of Boiling Water Steam See how the enthalpies of fusion and vaporization affect the shape of the curve and how superheating and supercooling affect the boiling and freezing points. Learn why boiling water stays at 100 °c (212 °f) no matter how much heat you add. Learn how to plot and interpret a heating curve for water, which shows the temperature changes as heat. What Is The Temperature Of Boiling Water Steam.
From labbyag.es
Water Boiling Temperature Pressure Chart Labb by AG What Is The Temperature Of Boiling Water Steam Learn how to plot and interpret a heating curve for water, which shows the temperature changes as heat is added to a sample of ice, water, and steam. The 'boiling point' of water is the temperature at which steam and liquid exist at equilibrium, and the roiling boil of a pot of water on the stove. Find out how boiling. What Is The Temperature Of Boiling Water Steam.
From chem.libretexts.org
11.7 Heating Curve for Water Chemistry LibreTexts What Is The Temperature Of Boiling Water Steam See the saturated steam table with properties like boiling point, specific volume,. See how the enthalpies of fusion and vaporization affect the shape of the curve and how superheating and supercooling affect the boiling and freezing points. 128 rows vacuum steam is the general term used for saturated steam at temperatures below 100°c. Learn how to plot and interpret a. What Is The Temperature Of Boiling Water Steam.
From mepacademy.com
Steam Heating System Basics MEP Academy What Is The Temperature Of Boiling Water Steam Boiling starts when it reaches 100 degree celsius and bulk vaporization takes place. These vaporised molecules, possessing same. Learn how to plot and interpret a heating curve for water, which shows the temperature changes as heat is added to a sample of ice, water, and steam. See how the enthalpies of fusion and vaporization affect the shape of the curve. What Is The Temperature Of Boiling Water Steam.
From yesikame.blogspot.com
Temperature Of Boiling Water / Proc Tech & Oper Acad Sensible & Latent What Is The Temperature Of Boiling Water Steam Boiling starts when it reaches 100 degree celsius and bulk vaporization takes place. See how the enthalpies of fusion and vaporization affect the shape of the curve and how superheating and supercooling affect the boiling and freezing points. Learn how to plot and interpret a heating curve for water, which shows the temperature changes as heat is added to a. What Is The Temperature Of Boiling Water Steam.
From sciencenotes.org
How to Boil Water at Room Temperature What Is The Temperature Of Boiling Water Steam See the saturated steam table with properties like boiling point, specific volume,. Boiling starts when it reaches 100 degree celsius and bulk vaporization takes place. The 'boiling point' of water is the temperature at which steam and liquid exist at equilibrium, and the roiling boil of a pot of water on the stove. 128 rows vacuum steam is the general. What Is The Temperature Of Boiling Water Steam.
From sciencenotes.org
Boiling Point Definition, Temperature, and Examples What Is The Temperature Of Boiling Water Steam See how the enthalpies of fusion and vaporization affect the shape of the curve and how superheating and supercooling affect the boiling and freezing points. Learn why boiling water stays at 100 °c (212 °f) no matter how much heat you add. 128 rows vacuum steam is the general term used for saturated steam at temperatures below 100°c. Find out. What Is The Temperature Of Boiling Water Steam.
From www.answerthehome.com
At What Temperature Does Water Steam? What Is The Temperature Of Boiling Water Steam See the saturated steam table with properties like boiling point, specific volume,. Boiling starts when it reaches 100 degree celsius and bulk vaporization takes place. Steam at atmospheric pressure is of limited practical use since it can not be conveyed by its own pressure along a steam pipe to. Find the density of saturated steam at different pressures in bar. What Is The Temperature Of Boiling Water Steam.
From gabriela-classwork.blogspot.com
Science Class septiembre 2010 What Is The Temperature Of Boiling Water Steam Find the density of saturated steam at different pressures in bar and temperature in celsius or fahrenheit. The 'boiling point' of water is the temperature at which steam and liquid exist at equilibrium, and the roiling boil of a pot of water on the stove. Find out how boiling point depends on pressure and what happens to water above and.. What Is The Temperature Of Boiling Water Steam.
From www.boiler-planning.com
Density Bosch Steam boiler planning Industrial Heat What Is The Temperature Of Boiling Water Steam Learn how to plot and interpret a heating curve for water, which shows the temperature changes as heat is added to a sample of ice, water, and steam. See how the enthalpies of fusion and vaporization affect the shape of the curve and how superheating and supercooling affect the boiling and freezing points. These vaporised molecules, possessing same. Learn why. What Is The Temperature Of Boiling Water Steam.
From www.youtube.com
How to measure oil and water boiling temperature? YouTube What Is The Temperature Of Boiling Water Steam Boiling starts when it reaches 100 degree celsius and bulk vaporization takes place. The 'boiling point' of water is the temperature at which steam and liquid exist at equilibrium, and the roiling boil of a pot of water on the stove. These vaporised molecules, possessing same. Learn why boiling water stays at 100 °c (212 °f) no matter how much. What Is The Temperature Of Boiling Water Steam.
From www.boiler-planning.com
Boiling pressure and temperature Bosch Steam boiler planning What Is The Temperature Of Boiling Water Steam Learn how to plot and interpret a heating curve for water, which shows the temperature changes as heat is added to a sample of ice, water, and steam. The 'boiling point' of water is the temperature at which steam and liquid exist at equilibrium, and the roiling boil of a pot of water on the stove. 128 rows vacuum steam. What Is The Temperature Of Boiling Water Steam.
From www.gkseries.com
Temperature of boiling water is measured by a ____ thermometer What Is The Temperature Of Boiling Water Steam Learn how to plot and interpret a heating curve for water, which shows the temperature changes as heat is added to a sample of ice, water, and steam. The 'boiling point' of water is the temperature at which steam and liquid exist at equilibrium, and the roiling boil of a pot of water on the stove. Find the density of. What Is The Temperature Of Boiling Water Steam.
From worldnewlive.com
At What Temperature Did The Water Start To Boil? Mastery Wiki What Is The Temperature Of Boiling Water Steam See the saturated steam table with properties like boiling point, specific volume,. See how the enthalpies of fusion and vaporization affect the shape of the curve and how superheating and supercooling affect the boiling and freezing points. 128 rows vacuum steam is the general term used for saturated steam at temperatures below 100°c. These vaporised molecules, possessing same. Boiling starts. What Is The Temperature Of Boiling Water Steam.
From ar.inspiredpencil.com
Boiling Point Of Water Examples What Is The Temperature Of Boiling Water Steam Find out how boiling point depends on pressure and what happens to water above and. Learn why boiling water stays at 100 °c (212 °f) no matter how much heat you add. These vaporised molecules, possessing same. Steam at atmospheric pressure is of limited practical use since it can not be conveyed by its own pressure along a steam pipe. What Is The Temperature Of Boiling Water Steam.
From www.boiler-planning.com
Steam Bosch Steam boiler planning Industrial Heat What Is The Temperature Of Boiling Water Steam Find out how boiling point depends on pressure and what happens to water above and. Learn how to plot and interpret a heating curve for water, which shows the temperature changes as heat is added to a sample of ice, water, and steam. These vaporised molecules, possessing same. See the saturated steam table with properties like boiling point, specific volume,.. What Is The Temperature Of Boiling Water Steam.
From www.vernier.com
Boiling Temperature of Water > Experiment 3 from Exploring Physical Science What Is The Temperature Of Boiling Water Steam Learn why boiling water stays at 100 °c (212 °f) no matter how much heat you add. See the saturated steam table with properties like boiling point, specific volume,. See how the enthalpies of fusion and vaporization affect the shape of the curve and how superheating and supercooling affect the boiling and freezing points. Steam at atmospheric pressure is of. What Is The Temperature Of Boiling Water Steam.
From www.shutterstock.com
Temperature Ice And Boiling Water Stock Vector 303198758 Shutterstock What Is The Temperature Of Boiling Water Steam Find the density of saturated steam at different pressures in bar and temperature in celsius or fahrenheit. Find out how boiling point depends on pressure and what happens to water above and. Boiling starts when it reaches 100 degree celsius and bulk vaporization takes place. Learn how to plot and interpret a heating curve for water, which shows the temperature. What Is The Temperature Of Boiling Water Steam.
From kenkidryer.com
Saturation temperature (boiling point) KENKI DRYER What Is The Temperature Of Boiling Water Steam Learn why boiling water stays at 100 °c (212 °f) no matter how much heat you add. Steam at atmospheric pressure is of limited practical use since it can not be conveyed by its own pressure along a steam pipe to. Find out how boiling point depends on pressure and what happens to water above and. These vaporised molecules, possessing. What Is The Temperature Of Boiling Water Steam.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? What Is The Temperature Of Boiling Water Steam 128 rows vacuum steam is the general term used for saturated steam at temperatures below 100°c. Find the density of saturated steam at different pressures in bar and temperature in celsius or fahrenheit. Find out how boiling point depends on pressure and what happens to water above and. Steam at atmospheric pressure is of limited practical use since it can. What Is The Temperature Of Boiling Water Steam.