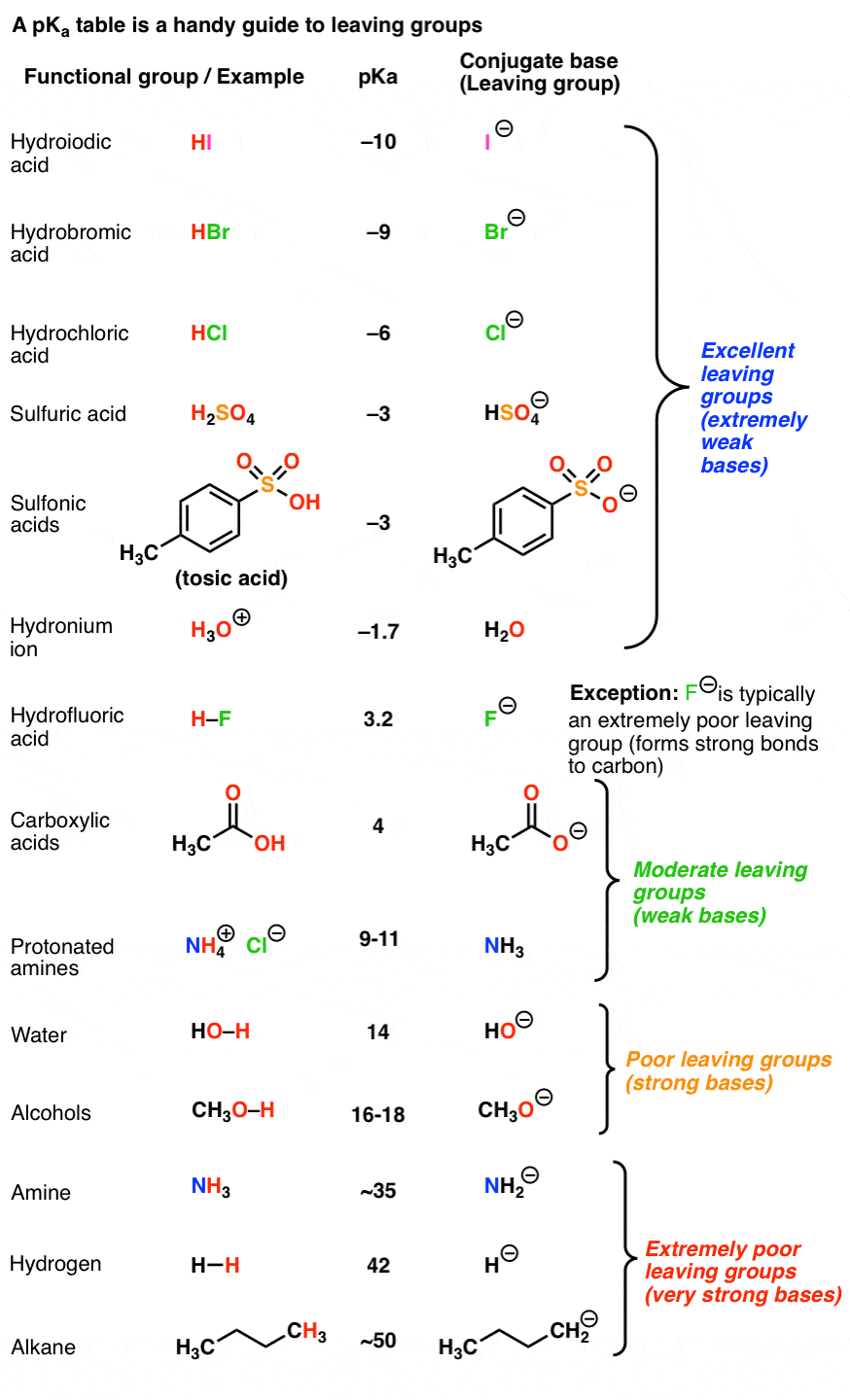
Bromine Vs Chlorine Leaving Group . If you take the example of the halogen family, fluorine is not a better leaving group. Poor leaving groups (which tend to be strong bases) can be made into better leaving groups through addition of a strong acid (or a lewis acid). Alkyl chlorides are indeed common reactants in laboratory nucleophilic substitution reactions, as are alkyl bromides and alkyl iodides. A better leaving group like bromine, and a weaker electron withdrawing group, tends to favor a concerted mechanism. In order for a leaving group to leave, it must be able to accept electrons. For example, alcohols can be converted into alkyl chlorides through addition of hcl. The best leaving groups want those electrons. A strong bases wants to donate electrons; They don't want to share them with other atoms. Good leaving groups are weak bases. As mentioned above, the more electronegative atoms may not be the better leaving group. The “original” reaction with a fluoride leaving group, and. Therefore, the leaving group must be a weak base.
from www.masterorganicchemistry.com
For example, alcohols can be converted into alkyl chlorides through addition of hcl. A strong bases wants to donate electrons; In order for a leaving group to leave, it must be able to accept electrons. Poor leaving groups (which tend to be strong bases) can be made into better leaving groups through addition of a strong acid (or a lewis acid). They don't want to share them with other atoms. Therefore, the leaving group must be a weak base. If you take the example of the halogen family, fluorine is not a better leaving group. A better leaving group like bromine, and a weaker electron withdrawing group, tends to favor a concerted mechanism. The best leaving groups want those electrons. As mentioned above, the more electronegative atoms may not be the better leaving group.
What makes a good leaving group? Master Organic Chemistry
Bromine Vs Chlorine Leaving Group As mentioned above, the more electronegative atoms may not be the better leaving group. Therefore, the leaving group must be a weak base. In order for a leaving group to leave, it must be able to accept electrons. Alkyl chlorides are indeed common reactants in laboratory nucleophilic substitution reactions, as are alkyl bromides and alkyl iodides. For example, alcohols can be converted into alkyl chlorides through addition of hcl. If you take the example of the halogen family, fluorine is not a better leaving group. Good leaving groups are weak bases. A strong bases wants to donate electrons; The best leaving groups want those electrons. As mentioned above, the more electronegative atoms may not be the better leaving group. They don't want to share them with other atoms. Poor leaving groups (which tend to be strong bases) can be made into better leaving groups through addition of a strong acid (or a lewis acid). The “original” reaction with a fluoride leaving group, and. A better leaving group like bromine, and a weaker electron withdrawing group, tends to favor a concerted mechanism.
From serumwatercare.com
Bromine vs. Chlorine Serum Watercare Bromine Vs Chlorine Leaving Group If you take the example of the halogen family, fluorine is not a better leaving group. As mentioned above, the more electronegative atoms may not be the better leaving group. Poor leaving groups (which tend to be strong bases) can be made into better leaving groups through addition of a strong acid (or a lewis acid). They don't want to. Bromine Vs Chlorine Leaving Group.
From bromineinformation.weebly.com
Bromine on the Periodic Table Bromine Vs Chlorine Leaving Group If you take the example of the halogen family, fluorine is not a better leaving group. The “original” reaction with a fluoride leaving group, and. Poor leaving groups (which tend to be strong bases) can be made into better leaving groups through addition of a strong acid (or a lewis acid). In order for a leaving group to leave, it. Bromine Vs Chlorine Leaving Group.
From thecontentauthority.com
Bromine vs Chlorine Do These Mean The Same? How To Use Them Bromine Vs Chlorine Leaving Group They don't want to share them with other atoms. The best leaving groups want those electrons. Alkyl chlorides are indeed common reactants in laboratory nucleophilic substitution reactions, as are alkyl bromides and alkyl iodides. If you take the example of the halogen family, fluorine is not a better leaving group. The “original” reaction with a fluoride leaving group, and. As. Bromine Vs Chlorine Leaving Group.
From blog.chloramineconsulting.com
Chlorine vs. Bromine in Indoor Pools Bromine Vs Chlorine Leaving Group Poor leaving groups (which tend to be strong bases) can be made into better leaving groups through addition of a strong acid (or a lewis acid). A better leaving group like bromine, and a weaker electron withdrawing group, tends to favor a concerted mechanism. Alkyl chlorides are indeed common reactants in laboratory nucleophilic substitution reactions, as are alkyl bromides and. Bromine Vs Chlorine Leaving Group.
From www.poolsuppliessuperstore.com
Bromine vs. Chlorine Everything You Need to Know Pool Supplies Bromine Vs Chlorine Leaving Group Poor leaving groups (which tend to be strong bases) can be made into better leaving groups through addition of a strong acid (or a lewis acid). As mentioned above, the more electronegative atoms may not be the better leaving group. Alkyl chlorides are indeed common reactants in laboratory nucleophilic substitution reactions, as are alkyl bromides and alkyl iodides. In order. Bromine Vs Chlorine Leaving Group.
From www.masterorganicchemistry.com
Selectivity in Free Radical Reactions Bromine vs. Chlorine — Master Bromine Vs Chlorine Leaving Group For example, alcohols can be converted into alkyl chlorides through addition of hcl. As mentioned above, the more electronegative atoms may not be the better leaving group. The “original” reaction with a fluoride leaving group, and. Good leaving groups are weak bases. If you take the example of the halogen family, fluorine is not a better leaving group. The best. Bromine Vs Chlorine Leaving Group.
From askanydifference.com
Bromine vs Chlorine Difference and Comparison Bromine Vs Chlorine Leaving Group A better leaving group like bromine, and a weaker electron withdrawing group, tends to favor a concerted mechanism. If you take the example of the halogen family, fluorine is not a better leaving group. Alkyl chlorides are indeed common reactants in laboratory nucleophilic substitution reactions, as are alkyl bromides and alkyl iodides. As mentioned above, the more electronegative atoms may. Bromine Vs Chlorine Leaving Group.
From www.slideshare.net
Lecture 6.1 The Periodic Table Bromine Vs Chlorine Leaving Group Alkyl chlorides are indeed common reactants in laboratory nucleophilic substitution reactions, as are alkyl bromides and alkyl iodides. For example, alcohols can be converted into alkyl chlorides through addition of hcl. If you take the example of the halogen family, fluorine is not a better leaving group. The best leaving groups want those electrons. As mentioned above, the more electronegative. Bromine Vs Chlorine Leaving Group.
From www.masterorganicchemistry.com
What makes a good leaving group? — Master Organic Chemistry Bromine Vs Chlorine Leaving Group As mentioned above, the more electronegative atoms may not be the better leaving group. A better leaving group like bromine, and a weaker electron withdrawing group, tends to favor a concerted mechanism. Good leaving groups are weak bases. Poor leaving groups (which tend to be strong bases) can be made into better leaving groups through addition of a strong acid. Bromine Vs Chlorine Leaving Group.
From blog.chloramineconsulting.com
Chlorine vs. Bromine in Indoor Pools Bromine Vs Chlorine Leaving Group For example, alcohols can be converted into alkyl chlorides through addition of hcl. Poor leaving groups (which tend to be strong bases) can be made into better leaving groups through addition of a strong acid (or a lewis acid). If you take the example of the halogen family, fluorine is not a better leaving group. A better leaving group like. Bromine Vs Chlorine Leaving Group.
From www.researchgate.net
(a) Electronic properties of iodine, bromine, and chlorine; (b Bromine Vs Chlorine Leaving Group If you take the example of the halogen family, fluorine is not a better leaving group. The best leaving groups want those electrons. Poor leaving groups (which tend to be strong bases) can be made into better leaving groups through addition of a strong acid (or a lewis acid). Alkyl chlorides are indeed common reactants in laboratory nucleophilic substitution reactions,. Bromine Vs Chlorine Leaving Group.
From askanydifference.com
Bromine vs Chlorine Difference and Comparison Bromine Vs Chlorine Leaving Group If you take the example of the halogen family, fluorine is not a better leaving group. For example, alcohols can be converted into alkyl chlorides through addition of hcl. Poor leaving groups (which tend to be strong bases) can be made into better leaving groups through addition of a strong acid (or a lewis acid). As mentioned above, the more. Bromine Vs Chlorine Leaving Group.
From www.masterorganicchemistry.com
What makes a good leaving group? Master Organic Chemistry Bromine Vs Chlorine Leaving Group Poor leaving groups (which tend to be strong bases) can be made into better leaving groups through addition of a strong acid (or a lewis acid). Alkyl chlorides are indeed common reactants in laboratory nucleophilic substitution reactions, as are alkyl bromides and alkyl iodides. The best leaving groups want those electrons. The “original” reaction with a fluoride leaving group, and.. Bromine Vs Chlorine Leaving Group.
From material-properties.org
Chlorine and Bromine Comparison Properties Material Properties Bromine Vs Chlorine Leaving Group In order for a leaving group to leave, it must be able to accept electrons. The “original” reaction with a fluoride leaving group, and. Therefore, the leaving group must be a weak base. For example, alcohols can be converted into alkyl chlorides through addition of hcl. Alkyl chlorides are indeed common reactants in laboratory nucleophilic substitution reactions, as are alkyl. Bromine Vs Chlorine Leaving Group.
From www.pinterest.com
Bromine vs. Chlorine in 2022 Pool maintenance, Pool, Chlorine Bromine Vs Chlorine Leaving Group The “original” reaction with a fluoride leaving group, and. Alkyl chlorides are indeed common reactants in laboratory nucleophilic substitution reactions, as are alkyl bromides and alkyl iodides. Therefore, the leaving group must be a weak base. They don't want to share them with other atoms. A strong bases wants to donate electrons; If you take the example of the halogen. Bromine Vs Chlorine Leaving Group.
From feedwater.co.uk
The Feedwater Knowledge base Important water treatment information Bromine Vs Chlorine Leaving Group Poor leaving groups (which tend to be strong bases) can be made into better leaving groups through addition of a strong acid (or a lewis acid). In order for a leaving group to leave, it must be able to accept electrons. As mentioned above, the more electronegative atoms may not be the better leaving group. They don't want to share. Bromine Vs Chlorine Leaving Group.
From www.diffzy.com
Bromine vs. Chlorine What's The Difference In Tabular Form, Points Bromine Vs Chlorine Leaving Group For example, alcohols can be converted into alkyl chlorides through addition of hcl. In order for a leaving group to leave, it must be able to accept electrons. A better leaving group like bromine, and a weaker electron withdrawing group, tends to favor a concerted mechanism. The best leaving groups want those electrons. Alkyl chlorides are indeed common reactants in. Bromine Vs Chlorine Leaving Group.
From resource.studiaacademy.com
Group 7 (Halogens) Chlorine, Bromine and Iodine Studia Academy Bromine Vs Chlorine Leaving Group Poor leaving groups (which tend to be strong bases) can be made into better leaving groups through addition of a strong acid (or a lewis acid). As mentioned above, the more electronegative atoms may not be the better leaving group. They don't want to share them with other atoms. Alkyl chlorides are indeed common reactants in laboratory nucleophilic substitution reactions,. Bromine Vs Chlorine Leaving Group.
From www.masterorganicchemistry.com
Selectivity in Free Radical Reactions Bromine vs. Chlorine — Master Bromine Vs Chlorine Leaving Group The best leaving groups want those electrons. Poor leaving groups (which tend to be strong bases) can be made into better leaving groups through addition of a strong acid (or a lewis acid). Good leaving groups are weak bases. A strong bases wants to donate electrons; Therefore, the leaving group must be a weak base. A better leaving group like. Bromine Vs Chlorine Leaving Group.
From www.slideserve.com
PPT Chemistry PowerPoint Presentation, free download ID1878282 Bromine Vs Chlorine Leaving Group The best leaving groups want those electrons. For example, alcohols can be converted into alkyl chlorides through addition of hcl. If you take the example of the halogen family, fluorine is not a better leaving group. As mentioned above, the more electronegative atoms may not be the better leaving group. A strong bases wants to donate electrons; Therefore, the leaving. Bromine Vs Chlorine Leaving Group.
From askanydifference.com
Bromine vs Chlorine Difference and Comparison Bromine Vs Chlorine Leaving Group The best leaving groups want those electrons. A better leaving group like bromine, and a weaker electron withdrawing group, tends to favor a concerted mechanism. They don't want to share them with other atoms. Good leaving groups are weak bases. In order for a leaving group to leave, it must be able to accept electrons. As mentioned above, the more. Bromine Vs Chlorine Leaving Group.
From www.slideserve.com
PPT iGCSE chemistry Section 2 lesson 2 PowerPoint Presentation, free Bromine Vs Chlorine Leaving Group Alkyl chlorides are indeed common reactants in laboratory nucleophilic substitution reactions, as are alkyl bromides and alkyl iodides. They don't want to share them with other atoms. The “original” reaction with a fluoride leaving group, and. As mentioned above, the more electronegative atoms may not be the better leaving group. The best leaving groups want those electrons. For example, alcohols. Bromine Vs Chlorine Leaving Group.
From www.masterorganicchemistry.com
What makes a good leaving group? Master Organic Chemistry Bromine Vs Chlorine Leaving Group In order for a leaving group to leave, it must be able to accept electrons. They don't want to share them with other atoms. Poor leaving groups (which tend to be strong bases) can be made into better leaving groups through addition of a strong acid (or a lewis acid). If you take the example of the halogen family, fluorine. Bromine Vs Chlorine Leaving Group.
From www.differencebetween.net
Difference Between Bromine and Chlorine Difference Between Bromine Vs Chlorine Leaving Group A strong bases wants to donate electrons; They don't want to share them with other atoms. Therefore, the leaving group must be a weak base. Alkyl chlorides are indeed common reactants in laboratory nucleophilic substitution reactions, as are alkyl bromides and alkyl iodides. As mentioned above, the more electronegative atoms may not be the better leaving group. Poor leaving groups. Bromine Vs Chlorine Leaving Group.
From www.researchgate.net
Scheme 6. Bromine migration using chlorine as directing group Bromine Vs Chlorine Leaving Group Alkyl chlorides are indeed common reactants in laboratory nucleophilic substitution reactions, as are alkyl bromides and alkyl iodides. As mentioned above, the more electronegative atoms may not be the better leaving group. If you take the example of the halogen family, fluorine is not a better leaving group. For example, alcohols can be converted into alkyl chlorides through addition of. Bromine Vs Chlorine Leaving Group.
From www.masterorganicchemistry.com
What makes a good leaving group? Master Organic Chemistry Bromine Vs Chlorine Leaving Group As mentioned above, the more electronegative atoms may not be the better leaving group. Therefore, the leaving group must be a weak base. Alkyl chlorides are indeed common reactants in laboratory nucleophilic substitution reactions, as are alkyl bromides and alkyl iodides. A better leaving group like bromine, and a weaker electron withdrawing group, tends to favor a concerted mechanism. If. Bromine Vs Chlorine Leaving Group.
From www.chegg.com
Solved I know Bromine is a better leaving group than Bromine Vs Chlorine Leaving Group A strong bases wants to donate electrons; Good leaving groups are weak bases. As mentioned above, the more electronegative atoms may not be the better leaving group. The best leaving groups want those electrons. The “original” reaction with a fluoride leaving group, and. Poor leaving groups (which tend to be strong bases) can be made into better leaving groups through. Bromine Vs Chlorine Leaving Group.
From www.ecospasalberta.com
A Comprehensive Comparison Bromine vs. Chlorine for Hot Tub Sanitation Bromine Vs Chlorine Leaving Group The best leaving groups want those electrons. The “original” reaction with a fluoride leaving group, and. A better leaving group like bromine, and a weaker electron withdrawing group, tends to favor a concerted mechanism. Good leaving groups are weak bases. For example, alcohols can be converted into alkyl chlorides through addition of hcl. Poor leaving groups (which tend to be. Bromine Vs Chlorine Leaving Group.
From blog.chloramineconsulting.com
Chlorine vs. Bromine in Indoor Pools Bromine Vs Chlorine Leaving Group For example, alcohols can be converted into alkyl chlorides through addition of hcl. As mentioned above, the more electronegative atoms may not be the better leaving group. Good leaving groups are weak bases. If you take the example of the halogen family, fluorine is not a better leaving group. They don't want to share them with other atoms. The best. Bromine Vs Chlorine Leaving Group.
From www.masterorganicchemistry.com
What makes a good leaving group? Master Organic Chemistry Bromine Vs Chlorine Leaving Group A better leaving group like bromine, and a weaker electron withdrawing group, tends to favor a concerted mechanism. Therefore, the leaving group must be a weak base. If you take the example of the halogen family, fluorine is not a better leaving group. As mentioned above, the more electronegative atoms may not be the better leaving group. For example, alcohols. Bromine Vs Chlorine Leaving Group.
From www.differencebetween.com
Difference Between Bromine and Chlorine Compare the Difference Bromine Vs Chlorine Leaving Group The best leaving groups want those electrons. Poor leaving groups (which tend to be strong bases) can be made into better leaving groups through addition of a strong acid (or a lewis acid). They don't want to share them with other atoms. A better leaving group like bromine, and a weaker electron withdrawing group, tends to favor a concerted mechanism.. Bromine Vs Chlorine Leaving Group.
From www.intec-america.com
Chlorine vs Bromine Which is Right Pool Sanitizer? Intec America Bromine Vs Chlorine Leaving Group The “original” reaction with a fluoride leaving group, and. Poor leaving groups (which tend to be strong bases) can be made into better leaving groups through addition of a strong acid (or a lewis acid). The best leaving groups want those electrons. Alkyl chlorides are indeed common reactants in laboratory nucleophilic substitution reactions, as are alkyl bromides and alkyl iodides.. Bromine Vs Chlorine Leaving Group.
From pediaa.com
Difference Between Bromine and Chlorine Bromine Vs Chlorine Leaving Group Therefore, the leaving group must be a weak base. The best leaving groups want those electrons. Poor leaving groups (which tend to be strong bases) can be made into better leaving groups through addition of a strong acid (or a lewis acid). For example, alcohols can be converted into alkyl chlorides through addition of hcl. They don't want to share. Bromine Vs Chlorine Leaving Group.
From www.masterorganicchemistry.com
Selectivity in Free Radical Reactions Bromine vs. Chlorine — Master Bromine Vs Chlorine Leaving Group A better leaving group like bromine, and a weaker electron withdrawing group, tends to favor a concerted mechanism. Alkyl chlorides are indeed common reactants in laboratory nucleophilic substitution reactions, as are alkyl bromides and alkyl iodides. The “original” reaction with a fluoride leaving group, and. Therefore, the leaving group must be a weak base. For example, alcohols can be converted. Bromine Vs Chlorine Leaving Group.
From www.masterorganicchemistry.com
Selectivity in Free Radical Reactions Bromine vs. Chlorine — Master Bromine Vs Chlorine Leaving Group Good leaving groups are weak bases. A strong bases wants to donate electrons; Therefore, the leaving group must be a weak base. A better leaving group like bromine, and a weaker electron withdrawing group, tends to favor a concerted mechanism. Poor leaving groups (which tend to be strong bases) can be made into better leaving groups through addition of a. Bromine Vs Chlorine Leaving Group.