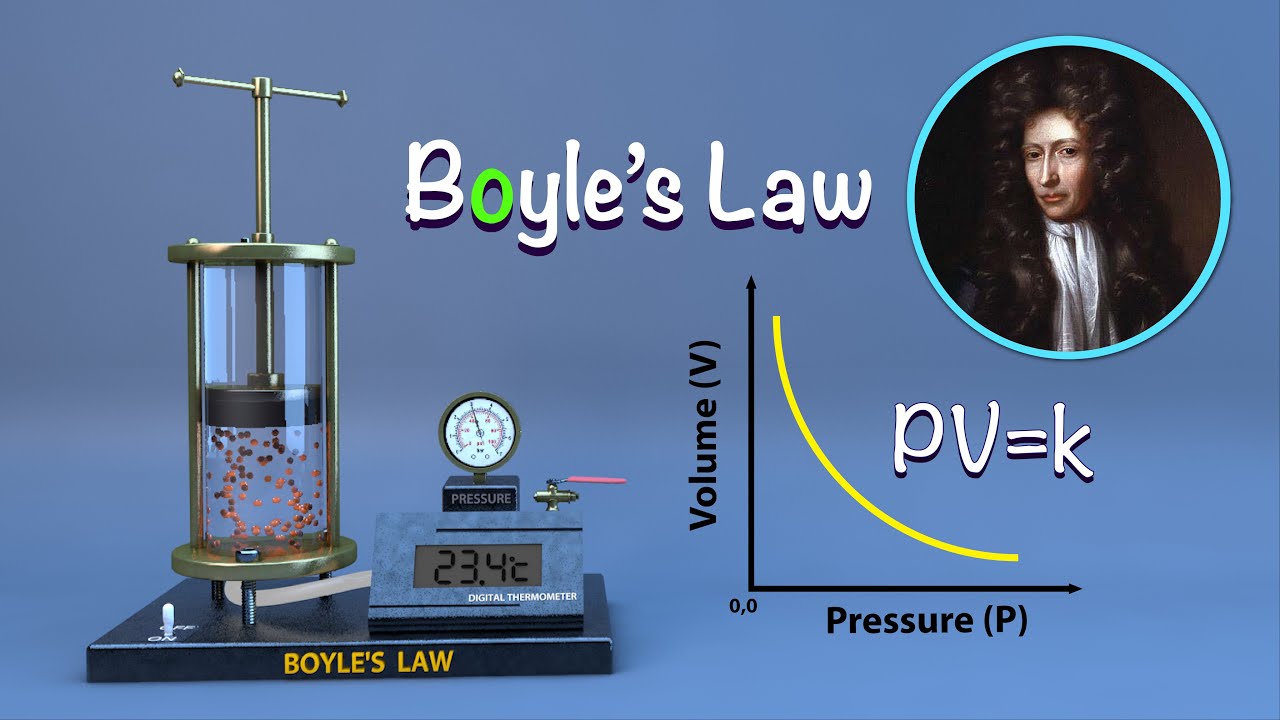
Boyle's Law Bike Tire . The simplest demonstration of boyle's law is a hand bicycle pump. When you pump air into a tire, the gas molecules. This law states that the pressure of a gas is inversely proportional to its volume at a constant temperature. The total volume of air that the tire can hold is directly related to the width of the tire. Boyles law intro and bike tire example. This is important, as you of course remember from high school, due to boyle’s law which basically says as volume. By pushing down on the piston, the reduced volume increases the pressure of the air. F will discuss the relationship between the. The volume (v) of an ideal gas varies inversely with the applied pressure (p) when the temperature (t) and the number of moles (n) of. When you compress the handle of a bike pump, you reduce the volume. The volume (v) of an ideal gas varies inversely with the applied pressure (p) when the temperature (t) and the number of moles (n) of the gas are constant.
from www.youtube.com
The simplest demonstration of boyle's law is a hand bicycle pump. When you pump air into a tire, the gas molecules. The volume (v) of an ideal gas varies inversely with the applied pressure (p) when the temperature (t) and the number of moles (n) of the gas are constant. Boyles law intro and bike tire example. This law states that the pressure of a gas is inversely proportional to its volume at a constant temperature. This is important, as you of course remember from high school, due to boyle’s law which basically says as volume. By pushing down on the piston, the reduced volume increases the pressure of the air. When you compress the handle of a bike pump, you reduce the volume. F will discuss the relationship between the. The volume (v) of an ideal gas varies inversely with the applied pressure (p) when the temperature (t) and the number of moles (n) of.
Boyle's law Explanation, Limitations and Applications Explained
Boyle's Law Bike Tire The total volume of air that the tire can hold is directly related to the width of the tire. The total volume of air that the tire can hold is directly related to the width of the tire. By pushing down on the piston, the reduced volume increases the pressure of the air. When you pump air into a tire, the gas molecules. When you compress the handle of a bike pump, you reduce the volume. F will discuss the relationship between the. This is important, as you of course remember from high school, due to boyle’s law which basically says as volume. The volume (v) of an ideal gas varies inversely with the applied pressure (p) when the temperature (t) and the number of moles (n) of the gas are constant. Boyles law intro and bike tire example. The volume (v) of an ideal gas varies inversely with the applied pressure (p) when the temperature (t) and the number of moles (n) of. The simplest demonstration of boyle's law is a hand bicycle pump. This law states that the pressure of a gas is inversely proportional to its volume at a constant temperature.
From www.slideserve.com
PPT GASES PowerPoint Presentation ID2912162 Boyle's Law Bike Tire When you compress the handle of a bike pump, you reduce the volume. The total volume of air that the tire can hold is directly related to the width of the tire. When you pump air into a tire, the gas molecules. The volume (v) of an ideal gas varies inversely with the applied pressure (p) when the temperature (t). Boyle's Law Bike Tire.
From slideplayer.com
Boyle’s Law P α 1/V This means Pressure and Volume are INVERSELY Boyle's Law Bike Tire The volume (v) of an ideal gas varies inversely with the applied pressure (p) when the temperature (t) and the number of moles (n) of the gas are constant. When you pump air into a tire, the gas molecules. This is important, as you of course remember from high school, due to boyle’s law which basically says as volume. This. Boyle's Law Bike Tire.
From www.slideserve.com
PPT BEHAVIOR OF GASES Chapter 12 PowerPoint Presentation, free Boyle's Law Bike Tire The simplest demonstration of boyle's law is a hand bicycle pump. When you compress the handle of a bike pump, you reduce the volume. The total volume of air that the tire can hold is directly related to the width of the tire. F will discuss the relationship between the. Boyles law intro and bike tire example. By pushing down. Boyle's Law Bike Tire.
From www.slideserve.com
PPT A Review PowerPoint Presentation, free download ID5767280 Boyle's Law Bike Tire This law states that the pressure of a gas is inversely proportional to its volume at a constant temperature. F will discuss the relationship between the. When you compress the handle of a bike pump, you reduce the volume. The total volume of air that the tire can hold is directly related to the width of the tire. The simplest. Boyle's Law Bike Tire.
From integrated-mcat.com
Integrated MCAT Course Boyle's Law Bike Tire When you pump air into a tire, the gas molecules. The total volume of air that the tire can hold is directly related to the width of the tire. This law states that the pressure of a gas is inversely proportional to its volume at a constant temperature. Boyles law intro and bike tire example. This is important, as you. Boyle's Law Bike Tire.
From manualworshipped.z14.web.core.windows.net
Principle Of Boyle's Law Boyle's Law Bike Tire This law states that the pressure of a gas is inversely proportional to its volume at a constant temperature. The total volume of air that the tire can hold is directly related to the width of the tire. This is important, as you of course remember from high school, due to boyle’s law which basically says as volume. The volume. Boyle's Law Bike Tire.
From ct-stem.northwestern.edu
CTSTEM Boyle's Law Bike Tire When you compress the handle of a bike pump, you reduce the volume. Boyles law intro and bike tire example. The volume (v) of an ideal gas varies inversely with the applied pressure (p) when the temperature (t) and the number of moles (n) of the gas are constant. This is important, as you of course remember from high school,. Boyle's Law Bike Tire.
From ar.inspiredpencil.com
Boyles Law Boyle's Law Bike Tire The simplest demonstration of boyle's law is a hand bicycle pump. The volume (v) of an ideal gas varies inversely with the applied pressure (p) when the temperature (t) and the number of moles (n) of. When you compress the handle of a bike pump, you reduce the volume. This law states that the pressure of a gas is inversely. Boyle's Law Bike Tire.
From www.slideserve.com
PPT Boyle’s Law and Charles’ Law PowerPoint Presentation, free Boyle's Law Bike Tire When you pump air into a tire, the gas molecules. The total volume of air that the tire can hold is directly related to the width of the tire. By pushing down on the piston, the reduced volume increases the pressure of the air. F will discuss the relationship between the. The volume (v) of an ideal gas varies inversely. Boyle's Law Bike Tire.
From www.numerade.com
"Identify the gas law that applies to the following scenario The tire Boyle's Law Bike Tire F will discuss the relationship between the. When you compress the handle of a bike pump, you reduce the volume. The volume (v) of an ideal gas varies inversely with the applied pressure (p) when the temperature (t) and the number of moles (n) of. This is important, as you of course remember from high school, due to boyle’s law. Boyle's Law Bike Tire.
From bikebesties.com
Explain How Pumping Air into a Bicycle Tire Increases the Pressure Boyle's Law Bike Tire This is important, as you of course remember from high school, due to boyle’s law which basically says as volume. The volume (v) of an ideal gas varies inversely with the applied pressure (p) when the temperature (t) and the number of moles (n) of. When you pump air into a tire, the gas molecules. The volume (v) of an. Boyle's Law Bike Tire.
From www.britannica.com
Boyle’s law Definition, Equation, & Facts Britannica Boyle's Law Bike Tire The simplest demonstration of boyle's law is a hand bicycle pump. The volume (v) of an ideal gas varies inversely with the applied pressure (p) when the temperature (t) and the number of moles (n) of. By pushing down on the piston, the reduced volume increases the pressure of the air. The total volume of air that the tire can. Boyle's Law Bike Tire.
From www.slideserve.com
PPT Gas Laws PowerPoint Presentation, free download ID2843041 Boyle's Law Bike Tire The volume (v) of an ideal gas varies inversely with the applied pressure (p) when the temperature (t) and the number of moles (n) of. This is important, as you of course remember from high school, due to boyle’s law which basically says as volume. F will discuss the relationship between the. Boyles law intro and bike tire example. This. Boyle's Law Bike Tire.
From www.slideserve.com
PPT GASES Chemistry I Honors Chapter 13 PowerPoint Presentation Boyle's Law Bike Tire By pushing down on the piston, the reduced volume increases the pressure of the air. This is important, as you of course remember from high school, due to boyle’s law which basically says as volume. This law states that the pressure of a gas is inversely proportional to its volume at a constant temperature. The volume (v) of an ideal. Boyle's Law Bike Tire.
From schematiclistbenz77.z13.web.core.windows.net
What Is Boyle's Law Simple Boyle's Law Bike Tire The simplest demonstration of boyle's law is a hand bicycle pump. This law states that the pressure of a gas is inversely proportional to its volume at a constant temperature. The total volume of air that the tire can hold is directly related to the width of the tire. The volume (v) of an ideal gas varies inversely with the. Boyle's Law Bike Tire.
From askfilo.com
1. You can observe a reallife application of Boyle's law when you fill y.. Boyle's Law Bike Tire The volume (v) of an ideal gas varies inversely with the applied pressure (p) when the temperature (t) and the number of moles (n) of the gas are constant. F will discuss the relationship between the. Boyles law intro and bike tire example. This law states that the pressure of a gas is inversely proportional to its volume at a. Boyle's Law Bike Tire.
From www.youtube.com
Boyles law intro and bike tire example YouTube Boyle's Law Bike Tire When you pump air into a tire, the gas molecules. This law states that the pressure of a gas is inversely proportional to its volume at a constant temperature. When you compress the handle of a bike pump, you reduce the volume. The volume (v) of an ideal gas varies inversely with the applied pressure (p) when the temperature (t). Boyle's Law Bike Tire.
From slideplayer.com
BEHAVIOR OF GASES Chapter ppt download Boyle's Law Bike Tire The volume (v) of an ideal gas varies inversely with the applied pressure (p) when the temperature (t) and the number of moles (n) of the gas are constant. The simplest demonstration of boyle's law is a hand bicycle pump. Boyles law intro and bike tire example. This is important, as you of course remember from high school, due to. Boyle's Law Bike Tire.
From www.sas.upenn.edu
Verification of Boyle's Law Boyle's Law Bike Tire F will discuss the relationship between the. This is important, as you of course remember from high school, due to boyle’s law which basically says as volume. Boyles law intro and bike tire example. The simplest demonstration of boyle's law is a hand bicycle pump. When you pump air into a tire, the gas molecules. By pushing down on the. Boyle's Law Bike Tire.
From schematicdiagramcactus.z13.web.core.windows.net
Explanation Of Boyle's Law Boyle's Law Bike Tire The volume (v) of an ideal gas varies inversely with the applied pressure (p) when the temperature (t) and the number of moles (n) of the gas are constant. When you pump air into a tire, the gas molecules. The simplest demonstration of boyle's law is a hand bicycle pump. By pushing down on the piston, the reduced volume increases. Boyle's Law Bike Tire.
From www.studypool.com
SOLUTION Boyle s law Studypool Boyle's Law Bike Tire By pushing down on the piston, the reduced volume increases the pressure of the air. When you pump air into a tire, the gas molecules. The simplest demonstration of boyle's law is a hand bicycle pump. This law states that the pressure of a gas is inversely proportional to its volume at a constant temperature. This is important, as you. Boyle's Law Bike Tire.
From mungfali.com
Boyle's Law Chart Boyle's Law Bike Tire The volume (v) of an ideal gas varies inversely with the applied pressure (p) when the temperature (t) and the number of moles (n) of the gas are constant. When you compress the handle of a bike pump, you reduce the volume. F will discuss the relationship between the. The simplest demonstration of boyle's law is a hand bicycle pump.. Boyle's Law Bike Tire.
From www.slideserve.com
PPT GASES Chemistry I Chapter 14 Chemistry I Honors Chapter 13 Boyle's Law Bike Tire The total volume of air that the tire can hold is directly related to the width of the tire. When you compress the handle of a bike pump, you reduce the volume. F will discuss the relationship between the. The simplest demonstration of boyle's law is a hand bicycle pump. When you pump air into a tire, the gas molecules.. Boyle's Law Bike Tire.
From www.pinterest.com
Boyle¡¯s Law infographic diagram with an example in a lab experiment Boyle's Law Bike Tire The total volume of air that the tire can hold is directly related to the width of the tire. Boyles law intro and bike tire example. The volume (v) of an ideal gas varies inversely with the applied pressure (p) when the temperature (t) and the number of moles (n) of. The simplest demonstration of boyle's law is a hand. Boyle's Law Bike Tire.
From melezy.com
Boyle's Law Definition, Equation, Examples, Calculator & FAQs Boyle's Law Bike Tire The volume (v) of an ideal gas varies inversely with the applied pressure (p) when the temperature (t) and the number of moles (n) of the gas are constant. By pushing down on the piston, the reduced volume increases the pressure of the air. This is important, as you of course remember from high school, due to boyle’s law which. Boyle's Law Bike Tire.
From slideplayer.com
With your lab partner, use the following scenarios as a guide to come Boyle's Law Bike Tire The volume (v) of an ideal gas varies inversely with the applied pressure (p) when the temperature (t) and the number of moles (n) of the gas are constant. Boyles law intro and bike tire example. When you pump air into a tire, the gas molecules. This law states that the pressure of a gas is inversely proportional to its. Boyle's Law Bike Tire.
From physicsinmyview.com
Boyle's Law Examples in Real Life (All New) Physics In My View Boyle's Law Bike Tire The volume (v) of an ideal gas varies inversely with the applied pressure (p) when the temperature (t) and the number of moles (n) of. Boyles law intro and bike tire example. F will discuss the relationship between the. When you pump air into a tire, the gas molecules. This law states that the pressure of a gas is inversely. Boyle's Law Bike Tire.
From www.studypool.com
SOLUTION Boyle s law with graphical representation Studypool Boyle's Law Bike Tire When you compress the handle of a bike pump, you reduce the volume. The volume (v) of an ideal gas varies inversely with the applied pressure (p) when the temperature (t) and the number of moles (n) of the gas are constant. This is important, as you of course remember from high school, due to boyle’s law which basically says. Boyle's Law Bike Tire.
From www.youtube.com
Bicycle pump application of Boyle’s Law in daily life. YouTube Boyle's Law Bike Tire The volume (v) of an ideal gas varies inversely with the applied pressure (p) when the temperature (t) and the number of moles (n) of the gas are constant. The simplest demonstration of boyle's law is a hand bicycle pump. The volume (v) of an ideal gas varies inversely with the applied pressure (p) when the temperature (t) and the. Boyle's Law Bike Tire.
From www.nagwa.com
Question Video Understanding Boyle’s Law Nagwa Boyle's Law Bike Tire The volume (v) of an ideal gas varies inversely with the applied pressure (p) when the temperature (t) and the number of moles (n) of the gas are constant. F will discuss the relationship between the. By pushing down on the piston, the reduced volume increases the pressure of the air. This is important, as you of course remember from. Boyle's Law Bike Tire.
From slideplayer.com
With your lab partner, use the following scenarios as a guide to come Boyle's Law Bike Tire The simplest demonstration of boyle's law is a hand bicycle pump. F will discuss the relationship between the. By pushing down on the piston, the reduced volume increases the pressure of the air. This is important, as you of course remember from high school, due to boyle’s law which basically says as volume. The volume (v) of an ideal gas. Boyle's Law Bike Tire.
From schematicdiagramcactus.z13.web.core.windows.net
What Is The Relationship Of Boyle's Law Boyle's Law Bike Tire The total volume of air that the tire can hold is directly related to the width of the tire. F will discuss the relationship between the. The volume (v) of an ideal gas varies inversely with the applied pressure (p) when the temperature (t) and the number of moles (n) of the gas are constant. By pushing down on the. Boyle's Law Bike Tire.
From www.youtube.com
Boyle's law Explanation, Limitations and Applications Explained Boyle's Law Bike Tire The total volume of air that the tire can hold is directly related to the width of the tire. F will discuss the relationship between the. This is important, as you of course remember from high school, due to boyle’s law which basically says as volume. The volume (v) of an ideal gas varies inversely with the applied pressure (p). Boyle's Law Bike Tire.
From www.fity.club
Boyle S Law Boyle's Law Bike Tire This is important, as you of course remember from high school, due to boyle’s law which basically says as volume. When you compress the handle of a bike pump, you reduce the volume. F will discuss the relationship between the. The simplest demonstration of boyle's law is a hand bicycle pump. By pushing down on the piston, the reduced volume. Boyle's Law Bike Tire.
From slideplayer.com
Foundations of Physics ppt download Boyle's Law Bike Tire By pushing down on the piston, the reduced volume increases the pressure of the air. The volume (v) of an ideal gas varies inversely with the applied pressure (p) when the temperature (t) and the number of moles (n) of the gas are constant. This law states that the pressure of a gas is inversely proportional to its volume at. Boyle's Law Bike Tire.