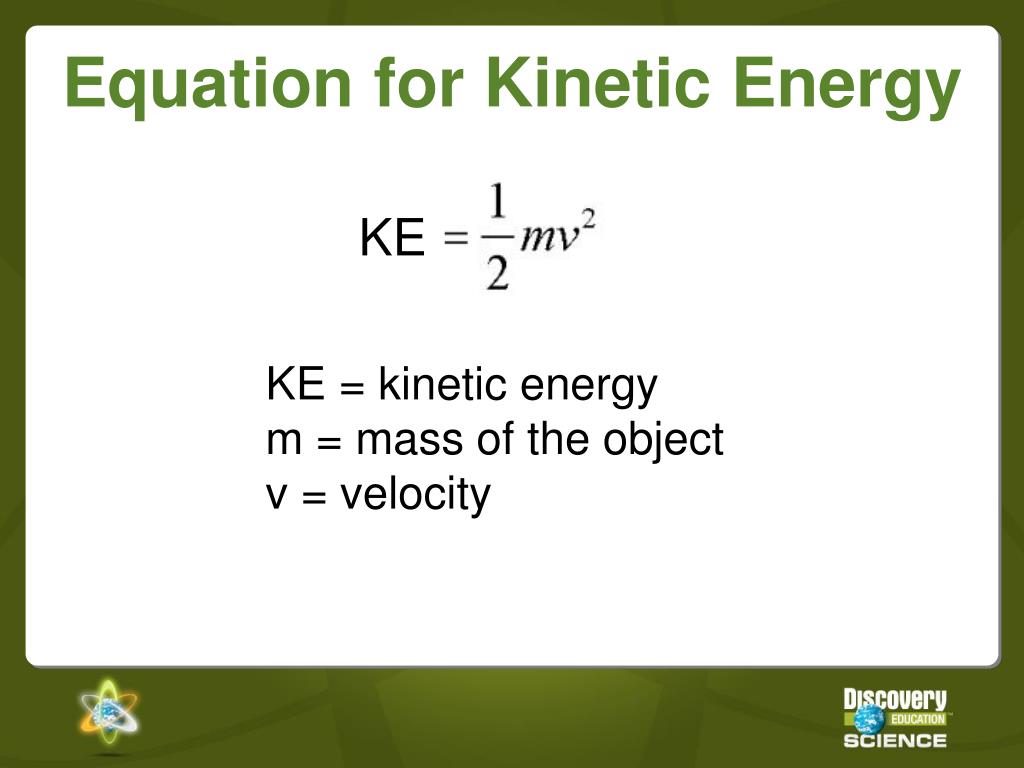
Is Internal Energy Kinetic Energy . The internal energy of a system is identified with the random, disordered motion of molecules; A reaction or process in which heat is transferred to a system from its surroundings is endothermic. The total (internal) energy in a system includes potential and kinetic energy. It is the total energy contained within the system. I.e., it is the sum of all types of energies possessed by a system. The first law of thermodynamics states that the energy of the universe is. The energy required by different. Internal energy is defined as the energy associated with the random, disordered motion of molecules. It is separated in scale from the. The change in internal energy. Internal energy is the sum of potential energy of the system and the system’s kinetic energy. Changes in a material's temperature or state of matter are caused by changes to the internal energy.
from www.slideserve.com
Changes in a material's temperature or state of matter are caused by changes to the internal energy. I.e., it is the sum of all types of energies possessed by a system. The energy required by different. Internal energy is the sum of potential energy of the system and the system’s kinetic energy. It is separated in scale from the. Internal energy is defined as the energy associated with the random, disordered motion of molecules. The total (internal) energy in a system includes potential and kinetic energy. The first law of thermodynamics states that the energy of the universe is. It is the total energy contained within the system. The internal energy of a system is identified with the random, disordered motion of molecules;
PPT Potential and Energy PowerPoint Presentation, free
Is Internal Energy Kinetic Energy The total (internal) energy in a system includes potential and kinetic energy. I.e., it is the sum of all types of energies possessed by a system. The total (internal) energy in a system includes potential and kinetic energy. The energy required by different. It is separated in scale from the. Internal energy is the sum of potential energy of the system and the system’s kinetic energy. It is the total energy contained within the system. The change in internal energy. Internal energy is defined as the energy associated with the random, disordered motion of molecules. Changes in a material's temperature or state of matter are caused by changes to the internal energy. The internal energy of a system is identified with the random, disordered motion of molecules; The first law of thermodynamics states that the energy of the universe is. A reaction or process in which heat is transferred to a system from its surroundings is endothermic.
From www.sciencelearn.org.nz
Potential and energy — Science Learning Hub Is Internal Energy Kinetic Energy The internal energy of a system is identified with the random, disordered motion of molecules; The total (internal) energy in a system includes potential and kinetic energy. A reaction or process in which heat is transferred to a system from its surroundings is endothermic. Internal energy is the sum of potential energy of the system and the system’s kinetic energy.. Is Internal Energy Kinetic Energy.
From ar.inspiredpencil.com
Energy Formula Units Is Internal Energy Kinetic Energy I.e., it is the sum of all types of energies possessed by a system. The internal energy of a system is identified with the random, disordered motion of molecules; A reaction or process in which heat is transferred to a system from its surroundings is endothermic. It is the total energy contained within the system. Internal energy is defined as. Is Internal Energy Kinetic Energy.
From www.researchgate.net
Conversion of energy and internal energy Download Scientific Is Internal Energy Kinetic Energy Internal energy is the sum of potential energy of the system and the system’s kinetic energy. It is the total energy contained within the system. The change in internal energy. It is separated in scale from the. The energy required by different. The internal energy of a system is identified with the random, disordered motion of molecules; The total (internal). Is Internal Energy Kinetic Energy.
From www.tes.com
Internal Energy Differentiated Lesson For Trilogy AQA Combined Science Is Internal Energy Kinetic Energy Changes in a material's temperature or state of matter are caused by changes to the internal energy. The change in internal energy. The internal energy of a system is identified with the random, disordered motion of molecules; A reaction or process in which heat is transferred to a system from its surroundings is endothermic. It is separated in scale from. Is Internal Energy Kinetic Energy.
From meaningkosh.com
Definition And Meaning In English MeaningKosh Is Internal Energy Kinetic Energy A reaction or process in which heat is transferred to a system from its surroundings is endothermic. The change in internal energy. I.e., it is the sum of all types of energies possessed by a system. The internal energy of a system is identified with the random, disordered motion of molecules; Internal energy is the sum of potential energy of. Is Internal Energy Kinetic Energy.
From mmerevise.co.uk
Thermal Energy Transfer Questions and Revision MME Is Internal Energy Kinetic Energy It is the total energy contained within the system. The total (internal) energy in a system includes potential and kinetic energy. The first law of thermodynamics states that the energy of the universe is. Internal energy is defined as the energy associated with the random, disordered motion of molecules. Changes in a material's temperature or state of matter are caused. Is Internal Energy Kinetic Energy.
From www.slideserve.com
PPT Potential and Energy PowerPoint Presentation, free Is Internal Energy Kinetic Energy It is separated in scale from the. Internal energy is the sum of potential energy of the system and the system’s kinetic energy. The energy required by different. The change in internal energy. The total (internal) energy in a system includes potential and kinetic energy. The internal energy of a system is identified with the random, disordered motion of molecules;. Is Internal Energy Kinetic Energy.
From www.researchgate.net
Histories of energy, internal energy, and their ratio of the Is Internal Energy Kinetic Energy Internal energy is the sum of potential energy of the system and the system’s kinetic energy. Changes in a material's temperature or state of matter are caused by changes to the internal energy. I.e., it is the sum of all types of energies possessed by a system. The total (internal) energy in a system includes potential and kinetic energy. The. Is Internal Energy Kinetic Energy.
From www.mechanicaleducation.com
Difference Between Energy And Potential Energy Mechanical Is Internal Energy Kinetic Energy Changes in a material's temperature or state of matter are caused by changes to the internal energy. The change in internal energy. It is the total energy contained within the system. The total (internal) energy in a system includes potential and kinetic energy. The internal energy of a system is identified with the random, disordered motion of molecules; It is. Is Internal Energy Kinetic Energy.
From www.thephysicsmill.com
Between the Two Shores Covalent Bonding Is Internal Energy Kinetic Energy The internal energy of a system is identified with the random, disordered motion of molecules; It is separated in scale from the. Changes in a material's temperature or state of matter are caused by changes to the internal energy. The change in internal energy. Internal energy is defined as the energy associated with the random, disordered motion of molecules. It. Is Internal Energy Kinetic Energy.
From www.teachoo.com
Energy Definition, Formula, Examples Teachoo Is Internal Energy Kinetic Energy The first law of thermodynamics states that the energy of the universe is. It is the total energy contained within the system. Changes in a material's temperature or state of matter are caused by changes to the internal energy. The internal energy of a system is identified with the random, disordered motion of molecules; Internal energy is the sum of. Is Internal Energy Kinetic Energy.
From byjus.com
The average internal energy of molecules of a substance is a Is Internal Energy Kinetic Energy It is the total energy contained within the system. The change in internal energy. Internal energy is defined as the energy associated with the random, disordered motion of molecules. I.e., it is the sum of all types of energies possessed by a system. Changes in a material's temperature or state of matter are caused by changes to the internal energy.. Is Internal Energy Kinetic Energy.
From quizizz.com
Energy Science Quizizz Is Internal Energy Kinetic Energy It is separated in scale from the. The internal energy of a system is identified with the random, disordered motion of molecules; The first law of thermodynamics states that the energy of the universe is. The total (internal) energy in a system includes potential and kinetic energy. It is the total energy contained within the system. The change in internal. Is Internal Energy Kinetic Energy.
From www.vrogue.co
Energy Definition Formula Examples Teachoo vrogue.co Is Internal Energy Kinetic Energy Internal energy is the sum of potential energy of the system and the system’s kinetic energy. I.e., it is the sum of all types of energies possessed by a system. It is the total energy contained within the system. Internal energy is defined as the energy associated with the random, disordered motion of molecules. It is separated in scale from. Is Internal Energy Kinetic Energy.
From www.slideserve.com
PPT Energy Transformation Vocabulary Unit 6 PowerPoint Presentation Is Internal Energy Kinetic Energy The internal energy of a system is identified with the random, disordered motion of molecules; Internal energy is defined as the energy associated with the random, disordered motion of molecules. Changes in a material's temperature or state of matter are caused by changes to the internal energy. The energy required by different. It is separated in scale from the. The. Is Internal Energy Kinetic Energy.
From www.researchgate.net
Internal energy, energy and total energy versus time Is Internal Energy Kinetic Energy It is separated in scale from the. The first law of thermodynamics states that the energy of the universe is. It is the total energy contained within the system. The internal energy of a system is identified with the random, disordered motion of molecules; Changes in a material's temperature or state of matter are caused by changes to the internal. Is Internal Energy Kinetic Energy.
From www.youtube.com
energy equation proof and explanation YouTube Is Internal Energy Kinetic Energy The change in internal energy. I.e., it is the sum of all types of energies possessed by a system. It is the total energy contained within the system. The first law of thermodynamics states that the energy of the universe is. The internal energy of a system is identified with the random, disordered motion of molecules; Changes in a material's. Is Internal Energy Kinetic Energy.
From www.slideserve.com
PPT Potential & Energy PowerPoint Presentation, free download Is Internal Energy Kinetic Energy The total (internal) energy in a system includes potential and kinetic energy. Internal energy is the sum of potential energy of the system and the system’s kinetic energy. The internal energy of a system is identified with the random, disordered motion of molecules; It is separated in scale from the. Changes in a material's temperature or state of matter are. Is Internal Energy Kinetic Energy.
From www.vecteezy.com
Potential and energy diagram. 27798551 Vector Art at Vecteezy Is Internal Energy Kinetic Energy I.e., it is the sum of all types of energies possessed by a system. The internal energy of a system is identified with the random, disordered motion of molecules; It is the total energy contained within the system. The energy required by different. The total (internal) energy in a system includes potential and kinetic energy. The first law of thermodynamics. Is Internal Energy Kinetic Energy.
From gbu-taganskij.ru
Energy Potential And Energy For Kids, 58 OFF Is Internal Energy Kinetic Energy The first law of thermodynamics states that the energy of the universe is. The internal energy of a system is identified with the random, disordered motion of molecules; A reaction or process in which heat is transferred to a system from its surroundings is endothermic. The total (internal) energy in a system includes potential and kinetic energy. Internal energy is. Is Internal Energy Kinetic Energy.
From online-learning-college.com
Gravitational potential and energy Calculating energies Is Internal Energy Kinetic Energy It is the total energy contained within the system. Internal energy is defined as the energy associated with the random, disordered motion of molecules. The change in internal energy. The internal energy of a system is identified with the random, disordered motion of molecules; The energy required by different. I.e., it is the sum of all types of energies possessed. Is Internal Energy Kinetic Energy.
From www.pinterest.it
This image shows a number of different examples where energy is Is Internal Energy Kinetic Energy A reaction or process in which heat is transferred to a system from its surroundings is endothermic. Internal energy is the sum of potential energy of the system and the system’s kinetic energy. I.e., it is the sum of all types of energies possessed by a system. Internal energy is defined as the energy associated with the random, disordered motion. Is Internal Energy Kinetic Energy.
From www.youtube.com
and Potential Energy Simplified YouTube Is Internal Energy Kinetic Energy The energy required by different. The total (internal) energy in a system includes potential and kinetic energy. A reaction or process in which heat is transferred to a system from its surroundings is endothermic. Internal energy is defined as the energy associated with the random, disordered motion of molecules. The internal energy of a system is identified with the random,. Is Internal Energy Kinetic Energy.
From ppt-online.org
theory of ideal gases презентация онлайн Is Internal Energy Kinetic Energy A reaction or process in which heat is transferred to a system from its surroundings is endothermic. The change in internal energy. The internal energy of a system is identified with the random, disordered motion of molecules; Internal energy is defined as the energy associated with the random, disordered motion of molecules. Internal energy is the sum of potential energy. Is Internal Energy Kinetic Energy.
From www.showme.com
Physics Potential, and Internal energy Science, Physics Is Internal Energy Kinetic Energy A reaction or process in which heat is transferred to a system from its surroundings is endothermic. The first law of thermodynamics states that the energy of the universe is. The internal energy of a system is identified with the random, disordered motion of molecules; The energy required by different. I.e., it is the sum of all types of energies. Is Internal Energy Kinetic Energy.
From www.youtube.com
Theory of Gases and Internal Energy YouTube Is Internal Energy Kinetic Energy It is the total energy contained within the system. The energy required by different. The total (internal) energy in a system includes potential and kinetic energy. Internal energy is the sum of potential energy of the system and the system’s kinetic energy. Changes in a material's temperature or state of matter are caused by changes to the internal energy. The. Is Internal Energy Kinetic Energy.
From www.tes.com
GCSEInternal energy Teaching Resources Is Internal Energy Kinetic Energy It is separated in scale from the. A reaction or process in which heat is transferred to a system from its surroundings is endothermic. I.e., it is the sum of all types of energies possessed by a system. It is the total energy contained within the system. The total (internal) energy in a system includes potential and kinetic energy. Internal. Is Internal Energy Kinetic Energy.
From www.slideserve.com
PPT Energy PowerPoint Presentation, free download ID6710377 Is Internal Energy Kinetic Energy I.e., it is the sum of all types of energies possessed by a system. The first law of thermodynamics states that the energy of the universe is. The internal energy of a system is identified with the random, disordered motion of molecules; It is separated in scale from the. The change in internal energy. It is the total energy contained. Is Internal Energy Kinetic Energy.
From www.pinterest.com
GCSE Physics Internal Energy and Specific Heat Capacity 27 Is Internal Energy Kinetic Energy The first law of thermodynamics states that the energy of the universe is. The total (internal) energy in a system includes potential and kinetic energy. The energy required by different. The internal energy of a system is identified with the random, disordered motion of molecules; I.e., it is the sum of all types of energies possessed by a system. It. Is Internal Energy Kinetic Energy.
From www.youtube.com
energy equation example IGCSE Physics YouTube Is Internal Energy Kinetic Energy The change in internal energy. Internal energy is the sum of potential energy of the system and the system’s kinetic energy. The energy required by different. I.e., it is the sum of all types of energies possessed by a system. The total (internal) energy in a system includes potential and kinetic energy. A reaction or process in which heat is. Is Internal Energy Kinetic Energy.
From kidsdiscover.com
Infographic Potential vs. Energy Kids Discover Is Internal Energy Kinetic Energy The change in internal energy. The total (internal) energy in a system includes potential and kinetic energy. The energy required by different. The first law of thermodynamics states that the energy of the universe is. I.e., it is the sum of all types of energies possessed by a system. A reaction or process in which heat is transferred to a. Is Internal Energy Kinetic Energy.
From www.hanlin.com
Edexcel A Level Physics复习笔记9.5 Temperature & Absolute Zero翰林国际教育 Is Internal Energy Kinetic Energy It is separated in scale from the. Changes in a material's temperature or state of matter are caused by changes to the internal energy. Internal energy is defined as the energy associated with the random, disordered motion of molecules. I.e., it is the sum of all types of energies possessed by a system. The change in internal energy. It is. Is Internal Energy Kinetic Energy.
From eduinput.com
Difference between Energy and Potential Energy Is Internal Energy Kinetic Energy It is the total energy contained within the system. Internal energy is the sum of potential energy of the system and the system’s kinetic energy. A reaction or process in which heat is transferred to a system from its surroundings is endothermic. The energy required by different. The total (internal) energy in a system includes potential and kinetic energy. The. Is Internal Energy Kinetic Energy.
From sciencenotes.org
What Is Energy? Energy Examples Is Internal Energy Kinetic Energy Internal energy is the sum of potential energy of the system and the system’s kinetic energy. It is separated in scale from the. The total (internal) energy in a system includes potential and kinetic energy. The energy required by different. Changes in a material's temperature or state of matter are caused by changes to the internal energy. It is the. Is Internal Energy Kinetic Energy.
From polarpedia.eu
energi Polarpedia Is Internal Energy Kinetic Energy Internal energy is defined as the energy associated with the random, disordered motion of molecules. It is the total energy contained within the system. The change in internal energy. The internal energy of a system is identified with the random, disordered motion of molecules; Changes in a material's temperature or state of matter are caused by changes to the internal. Is Internal Energy Kinetic Energy.