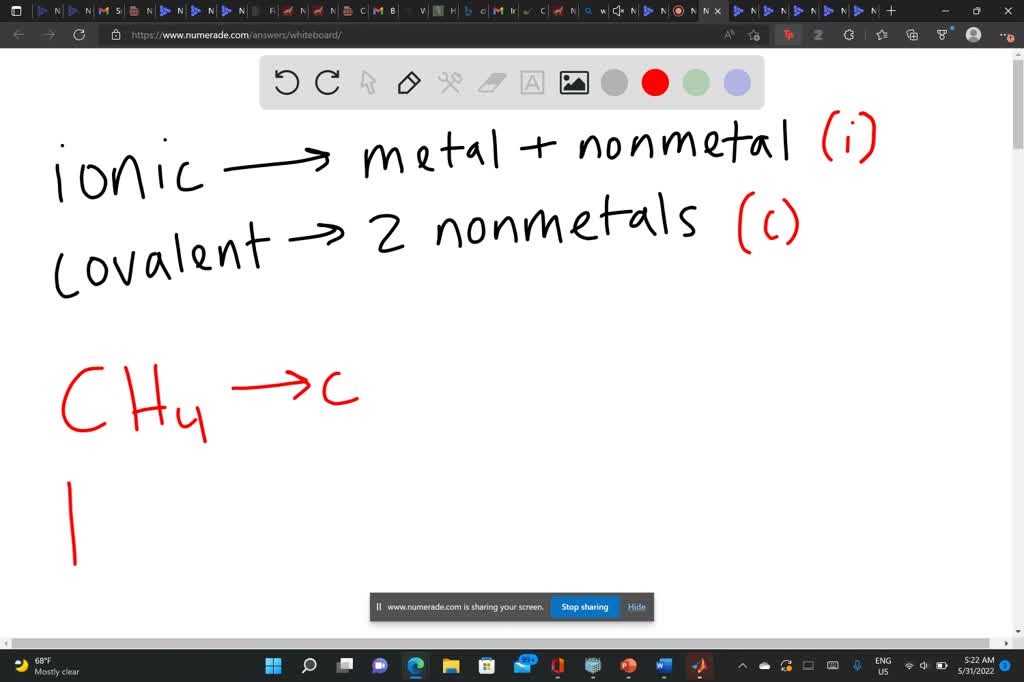
Magnesium Acetate Ionic Or Covalent . That is, does it have ionic bonds, or covalent bonds? Predict which forms an anion, which forms a cation, and the charges of each ion. Magnesium and nitrogen react to form an ionic compound. Physically, magnesium acetate appears as a white, crystalline substance that is highly soluble in water. Predict which forms an anion, which forms a cation, and the charges of each ion. Whereas ionic compounds are usually formed when a metal and a nonmetal combine, covalent compounds are usually formed by a combination. Magnesium and nitrogen react to form an ionic compound. Predict which forms an anion, which forms a cation, and the charges of each ion. Magnesium and nitrogen react to form an ionic compound. Write the symbol for each ion and name them. If it is covalent, which is typically. The first question we ask is if the compound is ionic or covalent?
from www.numerade.com
Write the symbol for each ion and name them. If it is covalent, which is typically. Physically, magnesium acetate appears as a white, crystalline substance that is highly soluble in water. Predict which forms an anion, which forms a cation, and the charges of each ion. Predict which forms an anion, which forms a cation, and the charges of each ion. Whereas ionic compounds are usually formed when a metal and a nonmetal combine, covalent compounds are usually formed by a combination. Predict which forms an anion, which forms a cation, and the charges of each ion. Magnesium and nitrogen react to form an ionic compound. The first question we ask is if the compound is ionic or covalent? That is, does it have ionic bonds, or covalent bonds?
SOLVED classify the following compounds as ionic or covalent compounds
Magnesium Acetate Ionic Or Covalent Predict which forms an anion, which forms a cation, and the charges of each ion. Predict which forms an anion, which forms a cation, and the charges of each ion. Magnesium and nitrogen react to form an ionic compound. Predict which forms an anion, which forms a cation, and the charges of each ion. That is, does it have ionic bonds, or covalent bonds? Whereas ionic compounds are usually formed when a metal and a nonmetal combine, covalent compounds are usually formed by a combination. Magnesium and nitrogen react to form an ionic compound. The first question we ask is if the compound is ionic or covalent? Physically, magnesium acetate appears as a white, crystalline substance that is highly soluble in water. If it is covalent, which is typically. Magnesium and nitrogen react to form an ionic compound. Write the symbol for each ion and name them. Predict which forms an anion, which forms a cation, and the charges of each ion.
From www.slideserve.com
PPT Chemical Formula and Naming PowerPoint Presentation, free Magnesium Acetate Ionic Or Covalent Predict which forms an anion, which forms a cation, and the charges of each ion. The first question we ask is if the compound is ionic or covalent? Magnesium and nitrogen react to form an ionic compound. Whereas ionic compounds are usually formed when a metal and a nonmetal combine, covalent compounds are usually formed by a combination. If it. Magnesium Acetate Ionic Or Covalent.
From courses.lumenlearning.com
Lewis Symbols and Structures CHEM 1305 Introductory Chemistry Magnesium Acetate Ionic Or Covalent Physically, magnesium acetate appears as a white, crystalline substance that is highly soluble in water. The first question we ask is if the compound is ionic or covalent? Write the symbol for each ion and name them. Predict which forms an anion, which forms a cation, and the charges of each ion. That is, does it have ionic bonds, or. Magnesium Acetate Ionic Or Covalent.
From triquetrahealth.com
Triquetra Health® Ionic Magnesium Liquid Supplement (8 oz) Triquetra™ Magnesium Acetate Ionic Or Covalent That is, does it have ionic bonds, or covalent bonds? If it is covalent, which is typically. Write the symbol for each ion and name them. The first question we ask is if the compound is ionic or covalent? Magnesium and nitrogen react to form an ionic compound. Predict which forms an anion, which forms a cation, and the charges. Magnesium Acetate Ionic Or Covalent.
From hamptonresearch.com
Hampton Research Magnesium Acetate Ionic Or Covalent Predict which forms an anion, which forms a cation, and the charges of each ion. Physically, magnesium acetate appears as a white, crystalline substance that is highly soluble in water. Magnesium and nitrogen react to form an ionic compound. Predict which forms an anion, which forms a cation, and the charges of each ion. The first question we ask is. Magnesium Acetate Ionic Or Covalent.
From www.slideserve.com
PPT Naming Ionic and Covalent Compounds PowerPoint Presentation, free Magnesium Acetate Ionic Or Covalent Magnesium and nitrogen react to form an ionic compound. The first question we ask is if the compound is ionic or covalent? Predict which forms an anion, which forms a cation, and the charges of each ion. That is, does it have ionic bonds, or covalent bonds? Magnesium and nitrogen react to form an ionic compound. Predict which forms an. Magnesium Acetate Ionic Or Covalent.
From studylib.net
Ionic Compounds Naming Magnesium Acetate Ionic Or Covalent Magnesium and nitrogen react to form an ionic compound. Predict which forms an anion, which forms a cation, and the charges of each ion. If it is covalent, which is typically. Physically, magnesium acetate appears as a white, crystalline substance that is highly soluble in water. Predict which forms an anion, which forms a cation, and the charges of each. Magnesium Acetate Ionic Or Covalent.
From studylib.net
Naming Ionic Compounds Answer Key Magnesium Acetate Ionic Or Covalent Predict which forms an anion, which forms a cation, and the charges of each ion. Predict which forms an anion, which forms a cation, and the charges of each ion. Magnesium and nitrogen react to form an ionic compound. Magnesium and nitrogen react to form an ionic compound. Magnesium and nitrogen react to form an ionic compound. If it is. Magnesium Acetate Ionic Or Covalent.
From www.youtube.com
How to Find the Ionic Charge for Magnesium (Mg) YouTube Magnesium Acetate Ionic Or Covalent If it is covalent, which is typically. Write the symbol for each ion and name them. Predict which forms an anion, which forms a cation, and the charges of each ion. Physically, magnesium acetate appears as a white, crystalline substance that is highly soluble in water. Whereas ionic compounds are usually formed when a metal and a nonmetal combine, covalent. Magnesium Acetate Ionic Or Covalent.
From www.numerade.com
SOLVED classify the following compounds as ionic or covalent compounds Magnesium Acetate Ionic Or Covalent Physically, magnesium acetate appears as a white, crystalline substance that is highly soluble in water. If it is covalent, which is typically. Write the symbol for each ion and name them. Whereas ionic compounds are usually formed when a metal and a nonmetal combine, covalent compounds are usually formed by a combination. Magnesium and nitrogen react to form an ionic. Magnesium Acetate Ionic Or Covalent.
From thefitnessmanual.com
Is Magnesium Nitrate Ionic Or Covalent TheFitnessManual Magnesium Acetate Ionic Or Covalent Predict which forms an anion, which forms a cation, and the charges of each ion. Whereas ionic compounds are usually formed when a metal and a nonmetal combine, covalent compounds are usually formed by a combination. Magnesium and nitrogen react to form an ionic compound. Magnesium and nitrogen react to form an ionic compound. That is, does it have ionic. Magnesium Acetate Ionic Or Covalent.
From www.vectorstock.com
Diagram representation of the element magnesium Vector Image Magnesium Acetate Ionic Or Covalent Magnesium and nitrogen react to form an ionic compound. Predict which forms an anion, which forms a cation, and the charges of each ion. Whereas ionic compounds are usually formed when a metal and a nonmetal combine, covalent compounds are usually formed by a combination. The first question we ask is if the compound is ionic or covalent? Predict which. Magnesium Acetate Ionic Or Covalent.
From www.calpaclab.com
Laboratory Chemicals, Magnesium Acetate, Tetrahydrate, Crystal, Reagent Magnesium Acetate Ionic Or Covalent Physically, magnesium acetate appears as a white, crystalline substance that is highly soluble in water. Predict which forms an anion, which forms a cation, and the charges of each ion. Magnesium and nitrogen react to form an ionic compound. If it is covalent, which is typically. The first question we ask is if the compound is ionic or covalent? Whereas. Magnesium Acetate Ionic Or Covalent.
From www.researchgate.net
Effects of magnesium acetate on the expression level of phosphorylated Magnesium Acetate Ionic Or Covalent Predict which forms an anion, which forms a cation, and the charges of each ion. The first question we ask is if the compound is ionic or covalent? Magnesium and nitrogen react to form an ionic compound. Whereas ionic compounds are usually formed when a metal and a nonmetal combine, covalent compounds are usually formed by a combination. Predict which. Magnesium Acetate Ionic Or Covalent.
From www.youtube.com
Draw the Lewis Structure of MgCl2 (magnesium chloride) YouTube Magnesium Acetate Ionic Or Covalent Magnesium and nitrogen react to form an ionic compound. Physically, magnesium acetate appears as a white, crystalline substance that is highly soluble in water. Predict which forms an anion, which forms a cation, and the charges of each ion. If it is covalent, which is typically. Write the symbol for each ion and name them. The first question we ask. Magnesium Acetate Ionic Or Covalent.
From dancorpexports.com
Top Export Import Company In Canada Dancorp Exports Magnesium Acetate Ionic Or Covalent Write the symbol for each ion and name them. Magnesium and nitrogen react to form an ionic compound. Physically, magnesium acetate appears as a white, crystalline substance that is highly soluble in water. Magnesium and nitrogen react to form an ionic compound. The first question we ask is if the compound is ionic or covalent? Magnesium and nitrogen react to. Magnesium Acetate Ionic Or Covalent.
From www.youtube.com
Is MgO (Magnesium oxide) Ionic or Covalent? YouTube Magnesium Acetate Ionic Or Covalent Predict which forms an anion, which forms a cation, and the charges of each ion. Physically, magnesium acetate appears as a white, crystalline substance that is highly soluble in water. Predict which forms an anion, which forms a cation, and the charges of each ion. Magnesium and nitrogen react to form an ionic compound. If it is covalent, which is. Magnesium Acetate Ionic Or Covalent.
From mungfali.com
PPT Section 9.1 Naming Ions PowerPoint Presentation Magnesium Acetate Ionic Or Covalent Magnesium and nitrogen react to form an ionic compound. If it is covalent, which is typically. Write the symbol for each ion and name them. Predict which forms an anion, which forms a cation, and the charges of each ion. Physically, magnesium acetate appears as a white, crystalline substance that is highly soluble in water. Whereas ionic compounds are usually. Magnesium Acetate Ionic Or Covalent.
From molekula.com
Purchase Magnesium acetate [142723] online • Catalog • Molekula Group Magnesium Acetate Ionic Or Covalent That is, does it have ionic bonds, or covalent bonds? Magnesium and nitrogen react to form an ionic compound. Physically, magnesium acetate appears as a white, crystalline substance that is highly soluble in water. Magnesium and nitrogen react to form an ionic compound. The first question we ask is if the compound is ionic or covalent? Predict which forms an. Magnesium Acetate Ionic Or Covalent.
From slidetodoc.com
Ionic Bonding Elements are the simplest substances There Magnesium Acetate Ionic Or Covalent The first question we ask is if the compound is ionic or covalent? Write the symbol for each ion and name them. Magnesium and nitrogen react to form an ionic compound. That is, does it have ionic bonds, or covalent bonds? Physically, magnesium acetate appears as a white, crystalline substance that is highly soluble in water. Magnesium and nitrogen react. Magnesium Acetate Ionic Or Covalent.
From www.youtube.com
Lewis Structure of Magnesium Oxide (MgO) YouTube Magnesium Acetate Ionic Or Covalent Magnesium and nitrogen react to form an ionic compound. Predict which forms an anion, which forms a cation, and the charges of each ion. Predict which forms an anion, which forms a cation, and the charges of each ion. Predict which forms an anion, which forms a cation, and the charges of each ion. Magnesium and nitrogen react to form. Magnesium Acetate Ionic Or Covalent.
From techiescientist.com
Is MgCl2 Ionic or Covalent? Techiescientist Magnesium Acetate Ionic Or Covalent If it is covalent, which is typically. Whereas ionic compounds are usually formed when a metal and a nonmetal combine, covalent compounds are usually formed by a combination. Predict which forms an anion, which forms a cation, and the charges of each ion. Predict which forms an anion, which forms a cation, and the charges of each ion. Magnesium and. Magnesium Acetate Ionic Or Covalent.
From hamptonresearch.com
Hampton Research Magnesium Acetate Ionic Or Covalent The first question we ask is if the compound is ionic or covalent? Predict which forms an anion, which forms a cation, and the charges of each ion. If it is covalent, which is typically. Magnesium and nitrogen react to form an ionic compound. Predict which forms an anion, which forms a cation, and the charges of each ion. Write. Magnesium Acetate Ionic Or Covalent.
From www.youtube.com
How to Write the Net Ionic Equation for Acetic acid + Sodium hydroxide Magnesium Acetate Ionic Or Covalent Write the symbol for each ion and name them. Magnesium and nitrogen react to form an ionic compound. That is, does it have ionic bonds, or covalent bonds? If it is covalent, which is typically. Predict which forms an anion, which forms a cation, and the charges of each ion. The first question we ask is if the compound is. Magnesium Acetate Ionic Or Covalent.
From techiescientist.com
Is MgO Ionic or Covalent? Techiescientist Magnesium Acetate Ionic Or Covalent Magnesium and nitrogen react to form an ionic compound. The first question we ask is if the compound is ionic or covalent? Write the symbol for each ion and name them. Magnesium and nitrogen react to form an ionic compound. Predict which forms an anion, which forms a cation, and the charges of each ion. Physically, magnesium acetate appears as. Magnesium Acetate Ionic Or Covalent.
From www.alamy.com
Diagram to show ionic bonding in magnesium oxide Stock Vector Image Magnesium Acetate Ionic Or Covalent Predict which forms an anion, which forms a cation, and the charges of each ion. Predict which forms an anion, which forms a cation, and the charges of each ion. That is, does it have ionic bonds, or covalent bonds? Magnesium and nitrogen react to form an ionic compound. If it is covalent, which is typically. Magnesium and nitrogen react. Magnesium Acetate Ionic Or Covalent.
From www.fishersci.com
Magnesium acetate, 1M aq. soln., Thermo Scientific Chemicals, Quantity Magnesium Acetate Ionic Or Covalent Predict which forms an anion, which forms a cation, and the charges of each ion. If it is covalent, which is typically. Magnesium and nitrogen react to form an ionic compound. That is, does it have ionic bonds, or covalent bonds? Physically, magnesium acetate appears as a white, crystalline substance that is highly soluble in water. Magnesium and nitrogen react. Magnesium Acetate Ionic Or Covalent.
From www.youtube.com
How to Write the Formula for Magnesium acetate YouTube Magnesium Acetate Ionic Or Covalent Predict which forms an anion, which forms a cation, and the charges of each ion. If it is covalent, which is typically. The first question we ask is if the compound is ionic or covalent? Magnesium and nitrogen react to form an ionic compound. Predict which forms an anion, which forms a cation, and the charges of each ion. Magnesium. Magnesium Acetate Ionic Or Covalent.
From www.fishersci.co.uk
Magnesium Acetate Tetrahydrate, Fisher BioReagents Poly Bottle; 500g Magnesium Acetate Ionic Or Covalent Magnesium and nitrogen react to form an ionic compound. Write the symbol for each ion and name them. Physically, magnesium acetate appears as a white, crystalline substance that is highly soluble in water. The first question we ask is if the compound is ionic or covalent? Magnesium and nitrogen react to form an ionic compound. If it is covalent, which. Magnesium Acetate Ionic Or Covalent.
From www.indiamart.com
Magnesium Acetate LR ACS at best price in Vadodara by Delta Scientific Magnesium Acetate Ionic Or Covalent The first question we ask is if the compound is ionic or covalent? If it is covalent, which is typically. Whereas ionic compounds are usually formed when a metal and a nonmetal combine, covalent compounds are usually formed by a combination. Magnesium and nitrogen react to form an ionic compound. Predict which forms an anion, which forms a cation, and. Magnesium Acetate Ionic Or Covalent.
From www.chemsrc.com
Magnesium silicate CAS1343880 Chemsrc Magnesium Acetate Ionic Or Covalent Magnesium and nitrogen react to form an ionic compound. The first question we ask is if the compound is ionic or covalent? Predict which forms an anion, which forms a cation, and the charges of each ion. Predict which forms an anion, which forms a cation, and the charges of each ion. Magnesium and nitrogen react to form an ionic. Magnesium Acetate Ionic Or Covalent.
From www.tessshebaylo.com
Net Ionic Equation Ammonium Bromide Dissolved In Water Tessshebaylo Magnesium Acetate Ionic Or Covalent Predict which forms an anion, which forms a cation, and the charges of each ion. Predict which forms an anion, which forms a cation, and the charges of each ion. If it is covalent, which is typically. Physically, magnesium acetate appears as a white, crystalline substance that is highly soluble in water. The first question we ask is if the. Magnesium Acetate Ionic Or Covalent.
From www.youtube.com
Magnesium carbonate YouTube Magnesium Acetate Ionic Or Covalent Predict which forms an anion, which forms a cation, and the charges of each ion. Magnesium and nitrogen react to form an ionic compound. The first question we ask is if the compound is ionic or covalent? If it is covalent, which is typically. Magnesium and nitrogen react to form an ionic compound. That is, does it have ionic bonds,. Magnesium Acetate Ionic Or Covalent.
From www.youtube.com
How to Draw the MgCl2 Lewis Dot Structure. YouTube Magnesium Acetate Ionic Or Covalent That is, does it have ionic bonds, or covalent bonds? Whereas ionic compounds are usually formed when a metal and a nonmetal combine, covalent compounds are usually formed by a combination. Physically, magnesium acetate appears as a white, crystalline substance that is highly soluble in water. Write the symbol for each ion and name them. Magnesium and nitrogen react to. Magnesium Acetate Ionic Or Covalent.
From www.youtube.com
Draw the Lewis Structure of MgBr2 (magnesium bromide) YouTube Magnesium Acetate Ionic Or Covalent The first question we ask is if the compound is ionic or covalent? Predict which forms an anion, which forms a cation, and the charges of each ion. Magnesium and nitrogen react to form an ionic compound. That is, does it have ionic bonds, or covalent bonds? Predict which forms an anion, which forms a cation, and the charges of. Magnesium Acetate Ionic Or Covalent.
From treatybottle13.pythonanywhere.com
Fun Word Equation For Magnesium And Oxygen Mlt Dimensions Table Magnesium Acetate Ionic Or Covalent Physically, magnesium acetate appears as a white, crystalline substance that is highly soluble in water. Whereas ionic compounds are usually formed when a metal and a nonmetal combine, covalent compounds are usually formed by a combination. That is, does it have ionic bonds, or covalent bonds? Predict which forms an anion, which forms a cation, and the charges of each. Magnesium Acetate Ionic Or Covalent.