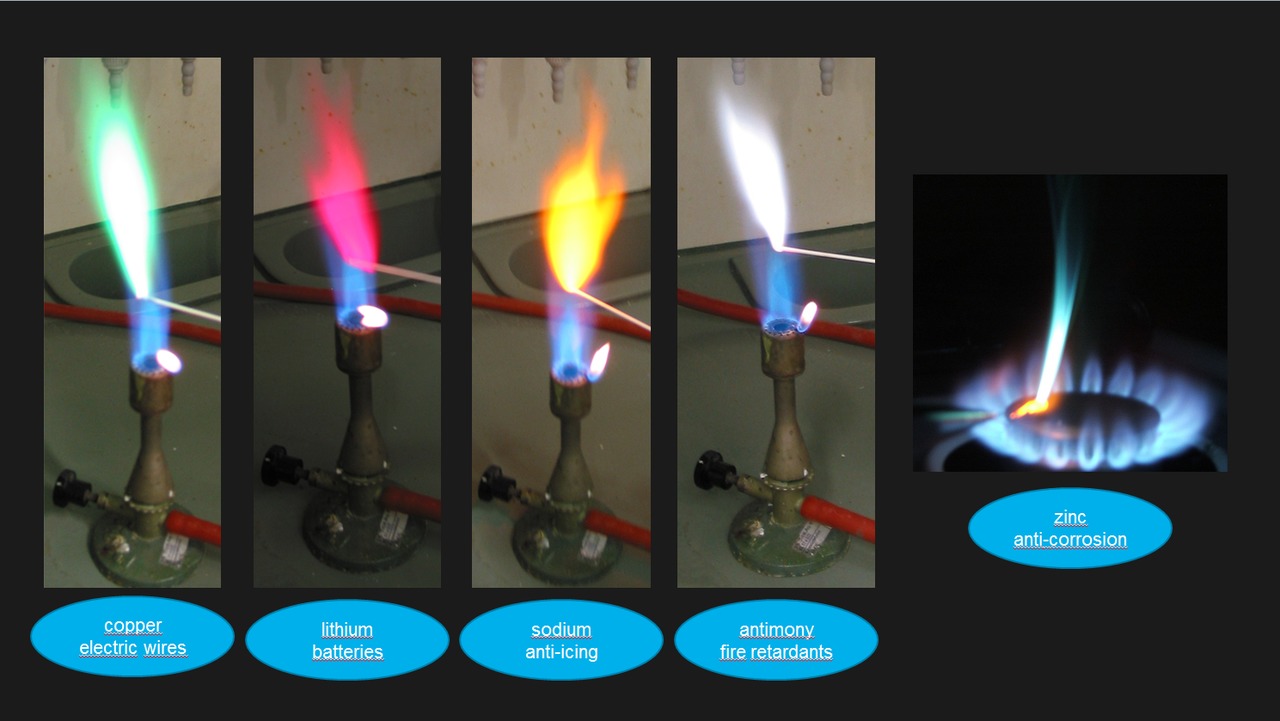
Copper Flame Colour . Flame tests are used to identify the presence of a relatively small number of metal ions in a compound. These flames can be used to produce atomic emmision spectra of the elements combusted. This page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. This is the basis of flame tests. A metal salt consists of a component cation (the metal) and an anion. Different metal electrons emit different wavelengths of light to return to their respective ground states, so the flame colors are varied. Copper colors a flame green, blue, or both depending on its oxidation state. For example, copper(i) emits blue light during the flame test, while copper(ii) emits green light. To carry out a flame test: Not all metal ions give flame colors. Copper(ii) produces a green flame. When heated in a flame, the element indium emits electromagnetic radiation with a distinctive indigo blue color (the name indium is derived from the word indigo). Ions produce different flame colours when they are heated strongly. The anion can affect the result of the flame test.
from ar.inspiredpencil.com
For example, copper(i) emits blue light during the flame test, while copper(ii) emits green light. This is the basis of flame tests. These flames can be used to produce atomic emmision spectra of the elements combusted. To carry out a flame test: When heated in a flame, the element indium emits electromagnetic radiation with a distinctive indigo blue color (the name indium is derived from the word indigo). A metal salt consists of a component cation (the metal) and an anion. Copper(ii) produces a green flame. Not all metal ions give flame colors. Ions produce different flame colours when they are heated strongly. Flame tests are used to identify the presence of a relatively small number of metal ions in a compound.
Copper Flame Color
Copper Flame Colour A metal salt consists of a component cation (the metal) and an anion. To carry out a flame test: A metal salt consists of a component cation (the metal) and an anion. Different metal electrons emit different wavelengths of light to return to their respective ground states, so the flame colors are varied. Copper colors a flame green, blue, or both depending on its oxidation state. Flame tests are used to identify the presence of a relatively small number of metal ions in a compound. Copper(ii) produces a green flame. This is the basis of flame tests. Ions produce different flame colours when they are heated strongly. These flames can be used to produce atomic emmision spectra of the elements combusted. When heated in a flame, the element indium emits electromagnetic radiation with a distinctive indigo blue color (the name indium is derived from the word indigo). This page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. The anion can affect the result of the flame test. Not all metal ions give flame colors. For example, copper(i) emits blue light during the flame test, while copper(ii) emits green light.
From www.pinterest.com
Copper Chloride Flame Radiant, Copper, Supplies Copper Flame Colour This page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. When heated in a flame, the element indium emits electromagnetic radiation with a distinctive indigo blue color (the name indium is derived from the word indigo). Not all metal ions give flame colors. These flames can be. Copper Flame Colour.
From chemistry.com.pk
Metal Ion Flame Test Colours [Infographic] Copper Flame Colour To carry out a flame test: When heated in a flame, the element indium emits electromagnetic radiation with a distinctive indigo blue color (the name indium is derived from the word indigo). Not all metal ions give flame colors. The anion can affect the result of the flame test. Copper(ii) produces a green flame. Flame tests are used to identify. Copper Flame Colour.
From mucholderthen.tumblr.com
Science Visualized • Flame color of various elements as compounds in... Copper Flame Colour A metal salt consists of a component cation (the metal) and an anion. Ions produce different flame colours when they are heated strongly. For example, copper(i) emits blue light during the flame test, while copper(ii) emits green light. The anion can affect the result of the flame test. When heated in a flame, the element indium emits electromagnetic radiation with. Copper Flame Colour.
From www.youtube.com
Flame Painting Copper YouTube Copper Flame Colour Copper(ii) produces a green flame. These flames can be used to produce atomic emmision spectra of the elements combusted. To carry out a flame test: The anion can affect the result of the flame test. Not all metal ions give flame colors. Flame tests are used to identify the presence of a relatively small number of metal ions in a. Copper Flame Colour.
From ar.inspiredpencil.com
Copper Flame Test Copper Flame Colour When heated in a flame, the element indium emits electromagnetic radiation with a distinctive indigo blue color (the name indium is derived from the word indigo). Ions produce different flame colours when they are heated strongly. Not all metal ions give flame colors. This page describes how to perform a flame test for a range of metal ions, and briefly. Copper Flame Colour.
From jewelrymakingjournal.com
Flame Painted Copper Jewelry Making Journal Copper Flame Colour For example, copper(i) emits blue light during the flame test, while copper(ii) emits green light. This page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. Different metal electrons emit different wavelengths of light to return to their respective ground states, so the flame colors are varied. To. Copper Flame Colour.
From www.youtube.com
Flame Painted Copper Using Real Fire on a pure copper canvas panel YouTube Copper Flame Colour Flame tests are used to identify the presence of a relatively small number of metal ions in a compound. Not all metal ions give flame colors. To carry out a flame test: Copper(ii) produces a green flame. Copper colors a flame green, blue, or both depending on its oxidation state. For example, copper(i) emits blue light during the flame test,. Copper Flame Colour.
From www.sciencephoto.com
Copper flame test Stock Image C024/3447 Science Photo Library Copper Flame Colour Different metal electrons emit different wavelengths of light to return to their respective ground states, so the flame colors are varied. The anion can affect the result of the flame test. Copper colors a flame green, blue, or both depending on its oxidation state. To carry out a flame test: Flame tests are used to identify the presence of a. Copper Flame Colour.
From sciencenotes.org
Flame Test Colors and Procedure (Chemistry) Copper Flame Colour These flames can be used to produce atomic emmision spectra of the elements combusted. This page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. The anion can affect the result of the flame test. A metal salt consists of a component cation (the metal) and an anion.. Copper Flame Colour.
From www.pinterest.com
Painting Colors onto Copper Using Only An Open Flame Make Copper uses, Fire Copper Flame Colour These flames can be used to produce atomic emmision spectra of the elements combusted. When heated in a flame, the element indium emits electromagnetic radiation with a distinctive indigo blue color (the name indium is derived from the word indigo). Ions produce different flame colours when they are heated strongly. Copper colors a flame green, blue, or both depending on. Copper Flame Colour.
From fphoto.photoshelter.com
science chemistry flame test Fundamental Photographs The Art of Science Copper Flame Colour This page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. Not all metal ions give flame colors. These flames can be used to produce atomic emmision spectra of the elements combusted. A metal salt consists of a component cation (the metal) and an anion. When heated in. Copper Flame Colour.
From www.basketofblue.com
How to Flame Paint Durable Colors on Copper Basket of Blue Copper Flame Colour Different metal electrons emit different wavelengths of light to return to their respective ground states, so the flame colors are varied. For example, copper(i) emits blue light during the flame test, while copper(ii) emits green light. This is the basis of flame tests. These flames can be used to produce atomic emmision spectra of the elements combusted. A metal salt. Copper Flame Colour.
From ar.inspiredpencil.com
Copper Flame Color Copper Flame Colour Not all metal ions give flame colors. Different metal electrons emit different wavelengths of light to return to their respective ground states, so the flame colors are varied. A metal salt consists of a component cation (the metal) and an anion. These flames can be used to produce atomic emmision spectra of the elements combusted. When heated in a flame,. Copper Flame Colour.
From www.thoughtco.com
Flame Test Colors Photo Gallery Copper Flame Colour When heated in a flame, the element indium emits electromagnetic radiation with a distinctive indigo blue color (the name indium is derived from the word indigo). For example, copper(i) emits blue light during the flame test, while copper(ii) emits green light. Different metal electrons emit different wavelengths of light to return to their respective ground states, so the flame colors. Copper Flame Colour.
From ar.inspiredpencil.com
Copper Flame Color Copper Flame Colour Different metal electrons emit different wavelengths of light to return to their respective ground states, so the flame colors are varied. Copper(ii) produces a green flame. This is the basis of flame tests. Ions produce different flame colours when they are heated strongly. Not all metal ions give flame colors. Flame tests are used to identify the presence of a. Copper Flame Colour.
From ar.inspiredpencil.com
Copper Flame Color Copper Flame Colour Not all metal ions give flame colors. Ions produce different flame colours when they are heated strongly. To carry out a flame test: These flames can be used to produce atomic emmision spectra of the elements combusted. Flame tests are used to identify the presence of a relatively small number of metal ions in a compound. When heated in a. Copper Flame Colour.
From sciencenotes.org
How to Make Colored Fire at Home Copper Flame Colour Flame tests are used to identify the presence of a relatively small number of metal ions in a compound. Copper colors a flame green, blue, or both depending on its oxidation state. The anion can affect the result of the flame test. To carry out a flame test: Different metal electrons emit different wavelengths of light to return to their. Copper Flame Colour.
From ar.inspiredpencil.com
Copper Flame Color Copper Flame Colour These flames can be used to produce atomic emmision spectra of the elements combusted. To carry out a flame test: When heated in a flame, the element indium emits electromagnetic radiation with a distinctive indigo blue color (the name indium is derived from the word indigo). Not all metal ions give flame colors. Different metal electrons emit different wavelengths of. Copper Flame Colour.
From ar.inspiredpencil.com
Copper Flame Color Copper Flame Colour Copper(ii) produces a green flame. This is the basis of flame tests. Flame tests are used to identify the presence of a relatively small number of metal ions in a compound. This page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. The anion can affect the result. Copper Flame Colour.
From www.youtube.com
Copper acetate flame test YouTube Copper Flame Colour Different metal electrons emit different wavelengths of light to return to their respective ground states, so the flame colors are varied. Not all metal ions give flame colors. A metal salt consists of a component cation (the metal) and an anion. For example, copper(i) emits blue light during the flame test, while copper(ii) emits green light. The anion can affect. Copper Flame Colour.
From www.youtube.com
Flame painted copper demo YouTube Copper Flame Colour Not all metal ions give flame colors. This page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. For example, copper(i) emits blue light during the flame test, while copper(ii) emits green light. This is the basis of flame tests. A metal salt consists of a component cation. Copper Flame Colour.
From www.alamy.com
A flame test for copper. Copper's compounds produce a bluegreen coloured flame when burnt Stock Copper Flame Colour To carry out a flame test: These flames can be used to produce atomic emmision spectra of the elements combusted. A metal salt consists of a component cation (the metal) and an anion. Not all metal ions give flame colors. Copper colors a flame green, blue, or both depending on its oxidation state. For example, copper(i) emits blue light during. Copper Flame Colour.
From www.basketofblue.com
How to Flame Paint Copper Basket of Blue Copper Flame Colour Ions produce different flame colours when they are heated strongly. When heated in a flame, the element indium emits electromagnetic radiation with a distinctive indigo blue color (the name indium is derived from the word indigo). To carry out a flame test: The anion can affect the result of the flame test. Copper(ii) produces a green flame. This is the. Copper Flame Colour.
From www.sciencephoto.com
Copper flame test Stock Image C024/3454 Science Photo Library Copper Flame Colour When heated in a flame, the element indium emits electromagnetic radiation with a distinctive indigo blue color (the name indium is derived from the word indigo). These flames can be used to produce atomic emmision spectra of the elements combusted. Ions produce different flame colours when they are heated strongly. Flame tests are used to identify the presence of a. Copper Flame Colour.
From ar.inspiredpencil.com
Copper Flame Color Copper Flame Colour For example, copper(i) emits blue light during the flame test, while copper(ii) emits green light. To carry out a flame test: Copper colors a flame green, blue, or both depending on its oxidation state. Ions produce different flame colours when they are heated strongly. Different metal electrons emit different wavelengths of light to return to their respective ground states, so. Copper Flame Colour.
From pixels.com
Flame Test Sequence Photograph by Science Photo Library Pixels Copper Flame Colour When heated in a flame, the element indium emits electromagnetic radiation with a distinctive indigo blue color (the name indium is derived from the word indigo). For example, copper(i) emits blue light during the flame test, while copper(ii) emits green light. A metal salt consists of a component cation (the metal) and an anion. Not all metal ions give flame. Copper Flame Colour.
From in.pinterest.com
Learn how to make colored fire at home in your fireplace or campfire. See which chemical produce Copper Flame Colour Not all metal ions give flame colors. These flames can be used to produce atomic emmision spectra of the elements combusted. Ions produce different flame colours when they are heated strongly. Flame tests are used to identify the presence of a relatively small number of metal ions in a compound. This page describes how to perform a flame test for. Copper Flame Colour.
From lostcowboys.com
Copper Flames Lost Cowboys Copper Flame Colour Flame tests are used to identify the presence of a relatively small number of metal ions in a compound. These flames can be used to produce atomic emmision spectra of the elements combusted. A metal salt consists of a component cation (the metal) and an anion. Ions produce different flame colours when they are heated strongly. This page describes how. Copper Flame Colour.
From www.pinterest.com
Flame painting copper Copper art, Copper patina, Fire painting Copper Flame Colour Different metal electrons emit different wavelengths of light to return to their respective ground states, so the flame colors are varied. A metal salt consists of a component cation (the metal) and an anion. The anion can affect the result of the flame test. Flame tests are used to identify the presence of a relatively small number of metal ions. Copper Flame Colour.
From www.flickr.com
Flame colours Copper chloride, Sodium chloride and Stronti… Flickr Copper Flame Colour For example, copper(i) emits blue light during the flame test, while copper(ii) emits green light. This is the basis of flame tests. Copper(ii) produces a green flame. This page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. Flame tests are used to identify the presence of a. Copper Flame Colour.
From evashermandesigns.blogspot.com
Eva Sherman Designs My First Large Scale Copper Flame Painting Copper Flame Colour Flame tests are used to identify the presence of a relatively small number of metal ions in a compound. To carry out a flame test: Copper(ii) produces a green flame. The anion can affect the result of the flame test. Not all metal ions give flame colors. For example, copper(i) emits blue light during the flame test, while copper(ii) emits. Copper Flame Colour.
From www.youtube.com
Flame Painting Copper Jewellery A Beginner’s Guide YouTube Copper Flame Colour Different metal electrons emit different wavelengths of light to return to their respective ground states, so the flame colors are varied. A metal salt consists of a component cation (the metal) and an anion. Flame tests are used to identify the presence of a relatively small number of metal ions in a compound. This page describes how to perform a. Copper Flame Colour.
From www.flickr.com
Copper flame color in methanol 2 photos of bluish green fl… Flickr Copper Flame Colour To carry out a flame test: This is the basis of flame tests. For example, copper(i) emits blue light during the flame test, while copper(ii) emits green light. Different metal electrons emit different wavelengths of light to return to their respective ground states, so the flame colors are varied. Copper(ii) produces a green flame. Ions produce different flame colours when. Copper Flame Colour.
From www.basketofblue.com
How to Flame Paint Copper Basket of Blue Copper Flame Colour Ions produce different flame colours when they are heated strongly. The anion can affect the result of the flame test. This page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. Not all metal ions give flame colors. Different metal electrons emit different wavelengths of light to return. Copper Flame Colour.
From ar.inspiredpencil.com
Copper Flame Color Copper Flame Colour When heated in a flame, the element indium emits electromagnetic radiation with a distinctive indigo blue color (the name indium is derived from the word indigo). Ions produce different flame colours when they are heated strongly. Not all metal ions give flame colors. Copper colors a flame green, blue, or both depending on its oxidation state. To carry out a. Copper Flame Colour.