Grams To Calorimeter . Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. Calorimetry is used to measure amounts of heat transferred to or from a substance. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Measure heat like a pro and unveil the secrets. When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. To do so, the heat is exchanged. The calorimetry calculator can help you solve complex calorimetry problems. How to calculate a calorimeter constant. To do so, the heat is exchanged with a calibrated object. Discover the hot science of calorimetry with our calorimetry calculator! To do so, the heat is exchanged with a calibrated object (calorimeter). Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Calorimetry is used to measure amounts of heat transferred to or from a substance. It can analyze the heat exchange between up to 3. Calorimetry is used to measure amounts of heat transferred to or from a substance.
from www.chegg.com
Measure heat like a pro and unveil the secrets. It can analyze the heat exchange between up to 3. Calorimetry is used to measure amounts of heat transferred to or from a substance. The calorimetry calculator can help you solve complex calorimetry problems. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. To do so, the heat is exchanged with a calibrated object (calorimeter). Discover the hot science of calorimetry with our calorimetry calculator! To do so, the heat is exchanged.
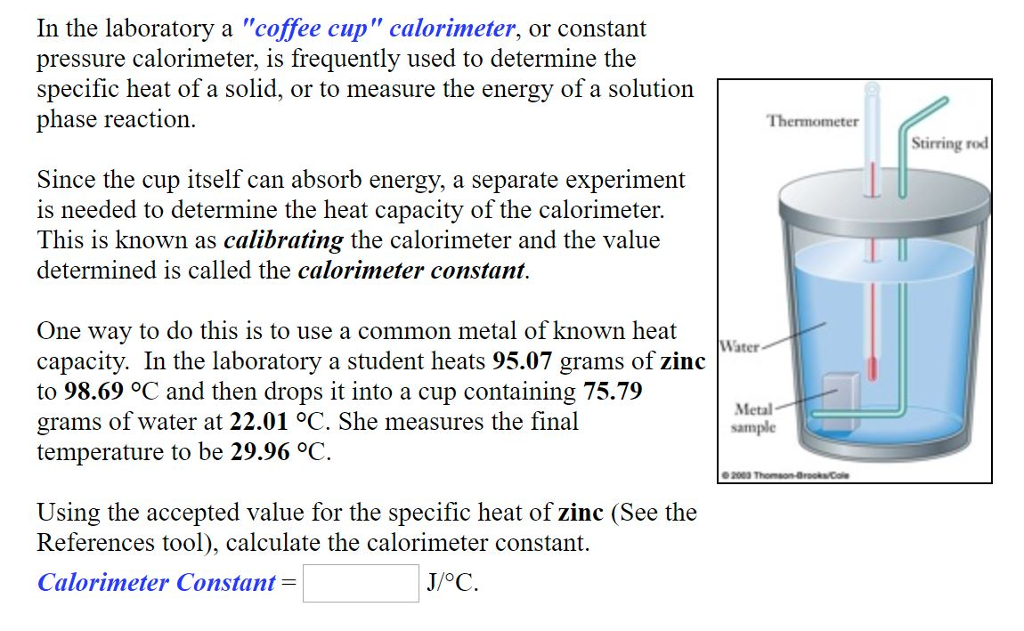
Solved In the laboratory a "coffee cup" calorimeter, or
Grams To Calorimeter Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. The calorimetry calculator can help you solve complex calorimetry problems. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Discover the hot science of calorimetry with our calorimetry calculator! Calorimetry is used to measure amounts of heat transferred to or from a substance. It can analyze the heat exchange between up to 3. Calorimetry is used to measure amounts of heat transferred to or from a substance. Measure heat like a pro and unveil the secrets. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged. How to calculate a calorimeter constant. To do so, the heat is exchanged with a calibrated object (calorimeter). To do so, the heat is exchanged with a calibrated object. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry.
From haipernews.com
How To Calculate Heat Capacity From Calorimeter Haiper Grams To Calorimeter Calorimetry is used to measure amounts of heat transferred to or from a substance. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. Discover the hot science of calorimetry with our calorimetry calculator! To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimetry is used to measure amounts of heat transferred to or. Grams To Calorimeter.
From askfilo.com
148 A calorimeter of mass 150 grams and specific heat 800 J/kg−K contains.. Grams To Calorimeter Measure heat like a pro and unveil the secrets. To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimetry is used to measure amounts of heat transferred to or from a substance. How to calculate a calorimeter constant. To do so, the heat is exchanged with a calibrated object. The calorimetry calculator can help you solve complex. Grams To Calorimeter.
From www.numerade.com
SOLVED A reaction of 0.25 grams of CaO(s) with excess HCl(aq) results Grams To Calorimeter Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. How to calculate a calorimeter constant. Discover the hot science of calorimetry with our calorimetry calculator! To do so, the heat is exchanged with a calibrated object. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry. Grams To Calorimeter.
From www.chegg.com
Solved In the laboratory a "coffee cup" calorimeter, or Grams To Calorimeter To do so, the heat is exchanged. Calorimetry is used to measure amounts of heat transferred to or from a substance. Measure heat like a pro and unveil the secrets. Calorimetry is used to measure amounts of heat transferred to or from a substance. It can analyze the heat exchange between up to 3. The calorimetry calculator can help you. Grams To Calorimeter.
From www.numerade.com
SOLVED 25.0 mL of 1.00 M HCl at 23.5°C react with 25.0 mL of 1.00 M Grams To Calorimeter The calorimetry calculator can help you solve complex calorimetry problems. It can analyze the heat exchange between up to 3. When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. To do so, the heat is exchanged with a calibrated object. How to calculate a calorimeter constant. Calorimetry is used. Grams To Calorimeter.
From www.numerade.com
When 2.0 grams of methane are burned in a bomb calorimeter containing Grams To Calorimeter Discover the hot science of calorimetry with our calorimetry calculator! Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. How to calculate a calorimeter constant. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so,. Grams To Calorimeter.
From www.numerade.com
SOLVED After heating 30 grams of an unknown metal to 100.0 *C it is Grams To Calorimeter Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. To do so, the heat is exchanged with a calibrated object. Measure heat like a pro and unveil the secrets. The calorimetry calculator can help you solve complex calorimetry problems. Calorimetry is used to measure amounts of heat transferred to or from. Grams To Calorimeter.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID1875569 Grams To Calorimeter Calorimetry is used to measure amounts of heat transferred to or from a substance. The calorimetry calculator can help you solve complex calorimetry problems. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. Calorimetry is used to measure amounts of heat transferred to or from a substance. It can analyze the heat exchange between up to. Grams To Calorimeter.
From www.youtube.com
Thermochemistry Enthalpy and Coffee Cup Calorimeter. YouTube Grams To Calorimeter Discover the hot science of calorimetry with our calorimetry calculator! The calorimetry calculator can help you solve complex calorimetry problems. To do so, the heat is exchanged. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is used to measure amounts of heat transferred to or from a substance. How to calculate a calorimeter. Grams To Calorimeter.
From www.youtube.com
How to find calorimeter constant YouTube Grams To Calorimeter The calorimetry calculator can help you solve complex calorimetry problems. To do so, the heat is exchanged. To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimetry is used to measure amounts of heat transferred to or from a substance. How to calculate a calorimeter constant. Calculate heat, temperature change, and specific heat after thermal equilibrium is. Grams To Calorimeter.
From www.numerade.com
SOLVED EXPERIMENT Calorimetry REPORT FORM Date 11/18/2021 Mass of Grams To Calorimeter How to calculate a calorimeter constant. To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimetry is used to measure amounts of heat transferred to or from a substance. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Calorimetry is used to measure amounts of heat transferred to. Grams To Calorimeter.
From www.toppr.com
A calorimeter of water equivalent 100 grams contains 200 grams of water Grams To Calorimeter Calorimetry is used to measure amounts of heat transferred to or from a substance. It can analyze the heat exchange between up to 3. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. Measure heat like a pro and unveil the secrets. When 40.0 ml of water at 60.0 °c is added to 40.0 ml at. Grams To Calorimeter.
From www.chegg.com
Solved Combustion (bomb) calorimeter. In an experiment, a Grams To Calorimeter Discover the hot science of calorimetry with our calorimetry calculator! Measure heat like a pro and unveil the secrets. When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. To do so, the heat is exchanged. To do so, the heat is exchanged with a calibrated object (calorimeter). Calculate heat,. Grams To Calorimeter.
From dokumen.tips
(PDF) · A bomb calorimeter containing 900 grams of water was calibrated Grams To Calorimeter To do so, the heat is exchanged. To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object. Compare heat. Grams To Calorimeter.
From www.numerade.com
SOLVED Need help calculating the calorimeter constant with the answer Grams To Calorimeter Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is used to measure amounts of heat transferred to or from a substance. It can analyze the heat exchange between up to 3. Measure heat like a pro and unveil the secrets. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry.. Grams To Calorimeter.
From www.numerade.com
SOLVED A 44.0 g sample of an unknown metal at 99.0 °C was placed in a Grams To Calorimeter Measure heat like a pro and unveil the secrets. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object. The calorimetry calculator can help you solve complex calorimetry problems. Calorimetry is. Grams To Calorimeter.
From www.numerade.com
When 1.681 grams of sucrose (Molar mass 342.3 g/mol) is burned in a Grams To Calorimeter Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Measure heat like a pro and unveil the secrets. Calorimetry is used to measure amounts of heat transferred to or from a substance. The calorimetry calculator can help you solve complex calorimetry problems. Calculate the molar heat of enthalpy for a reactions using coffee. Grams To Calorimeter.
From www.chegg.com
Solved Use the References to access important values if Grams To Calorimeter The calorimetry calculator can help you solve complex calorimetry problems. It can analyze the heat exchange between up to 3. Measure heat like a pro and unveil the secrets. Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged. To do so, the heat is exchanged with a calibrated. Grams To Calorimeter.
From guernseydonkey.com
How is the Caloric Value of Food Measured? Grams To Calorimeter To do so, the heat is exchanged. It can analyze the heat exchange between up to 3. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is used to measure amounts of heat transferred to or. Grams To Calorimeter.
From www.numerade.com
SOLVED In the laboratory a "coffee cup" calorimeter, or constant Grams To Calorimeter Discover the hot science of calorimetry with our calorimetry calculator! When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. It can analyze the heat exchange between up to 3. Measure heat like a pro and unveil. Grams To Calorimeter.
From www.chegg.com
Solved In the laboratory a "coffee cup" calorimeter, or Grams To Calorimeter Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. To do so, the heat is exchanged with a calibrated object. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. The calorimetry. Grams To Calorimeter.
From askfilo.com
2. Twenty grams of copper at 60∘C are placed in an insulated calorimeter Grams To Calorimeter It can analyze the heat exchange between up to 3. Measure heat like a pro and unveil the secrets. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between. Grams To Calorimeter.
From www.numerade.com
SOLVED In the laboratory a "coffee cup calorimeter, Or constant Grams To Calorimeter Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object (calorimeter). It can analyze the heat exchange between up to 3. The calorimetry calculator can help you solve complex calorimetry problems. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. Calorimetry. Grams To Calorimeter.
From www.numerade.com
SOLVED In the laboratory a "coffee cup" calorimeter, or constant Grams To Calorimeter It can analyze the heat exchange between up to 3. Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. Measure heat like a pro and unveil the secrets. To do so, the heat is exchanged. Grams To Calorimeter.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Grams To Calorimeter It can analyze the heat exchange between up to 3. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. To do so, the heat is exchanged with a calibrated object (calorimeter). Discover the hot science of. Grams To Calorimeter.
From www.numerade.com
SOLVED In the laboratory a "coffee cup" calorimeter, Or constant Grams To Calorimeter Discover the hot science of calorimetry with our calorimetry calculator! To do so, the heat is exchanged with a calibrated object (calorimeter). It can analyze the heat exchange between up to 3. When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. Calorimetry is used to measure amounts of heat. Grams To Calorimeter.
From www.numerade.com
SOLVED A 0.750kilogram calorimeter, with an unknown specific heat Grams To Calorimeter Measure heat like a pro and unveil the secrets. How to calculate a calorimeter constant. Discover the hot science of calorimetry with our calorimetry calculator! To do so, the heat is exchanged with a calibrated object (calorimeter). Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. To do so, the heat. Grams To Calorimeter.
From www.chegg.com
Solved When 2.0 grams of methane are burned in a bomb Grams To Calorimeter To do so, the heat is exchanged. To do so, the heat is exchanged with a calibrated object (calorimeter). Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. How to calculate a calorimeter constant. Calorimetry is used to measure amounts of heat transferred to or from a substance. Discover the hot science of. Grams To Calorimeter.
From www.chegg.com
Solved A bomb calorimeter, or constant volume calorimeter, Grams To Calorimeter How to calculate a calorimeter constant. Measure heat like a pro and unveil the secrets. Calorimetry is used to measure amounts of heat transferred to or from a substance. It can analyze the heat exchange between up to 3. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is used to measure amounts of. Grams To Calorimeter.
From www.chegg.com
or this part of the lab, you will analyze recorded Grams To Calorimeter Calorimetry is used to measure amounts of heat transferred to or from a substance. When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. The calorimetry calculator can help you solve complex calorimetry problems. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calculate heat,. Grams To Calorimeter.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube Grams To Calorimeter When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. It can analyze the heat exchange between up to 3. To do so, the heat is exchanged with a calibrated object (calorimeter). Discover the hot science of calorimetry with our calorimetry calculator! Calculate the molar heat of enthalpy for a. Grams To Calorimeter.
From www.chegg.com
Solved A student has a calorimeter with 211.7 grams of Grams To Calorimeter How to calculate a calorimeter constant. Calorimetry is used to measure amounts of heat transferred to or from a substance. When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. Discover the hot science of calorimetry with our calorimetry calculator! Calculate the molar heat of enthalpy for a reactions using. Grams To Calorimeter.
From www.chegg.com
Solved In the laboratory a "coffee cup" calorimeter, or Grams To Calorimeter Discover the hot science of calorimetry with our calorimetry calculator! It can analyze the heat exchange between up to 3. Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object (calorimeter). The calorimetry calculator can help you solve complex calorimetry problems. Calculate the molar heat. Grams To Calorimeter.
From www.chegg.com
Solved In The Laboratory A "coffee Cup" Calorimeter, Or C... Grams To Calorimeter It can analyze the heat exchange between up to 3. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. The calorimetry calculator can help you solve complex calorimetry problems. When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. To do so, the heat is exchanged.. Grams To Calorimeter.
From www.chegg.com
Solved Thermometer A bomb calorimeter, or constant volume Grams To Calorimeter Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. How to calculate a calorimeter constant. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. To do so,. Grams To Calorimeter.