Magnesium Electrons Lose To Become Stable . Magnesium loses two electrons when reacting. Magnesium (mg) has the electron arrangement 2,8,2. This is because it is in the second group of the periodic table, and elements in this. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the. If an element is located on the left side of the table (metal) and has less than three valence electrons, it will lose its valence in order to become stable and achieve an. Magnesium loses electrons to become an ion. To become stable it must lose its two outer electrons to obtain a full outer. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. One way they can achieve this is by gaining or losing electrons. A reactive complex has finally been made in which magnesium keeps all of its electrons, and which. Magnesium atoms typically lose two electrons to form chemical compounds.
from slidetodoc.com
Magnesium loses electrons to become an ion. If an element is located on the left side of the table (metal) and has less than three valence electrons, it will lose its valence in order to become stable and achieve an. One way they can achieve this is by gaining or losing electrons. Magnesium loses two electrons when reacting. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the. Magnesium atoms typically lose two electrons to form chemical compounds. Magnesium (mg) has the electron arrangement 2,8,2. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. This is because it is in the second group of the periodic table, and elements in this. A reactive complex has finally been made in which magnesium keeps all of its electrons, and which.
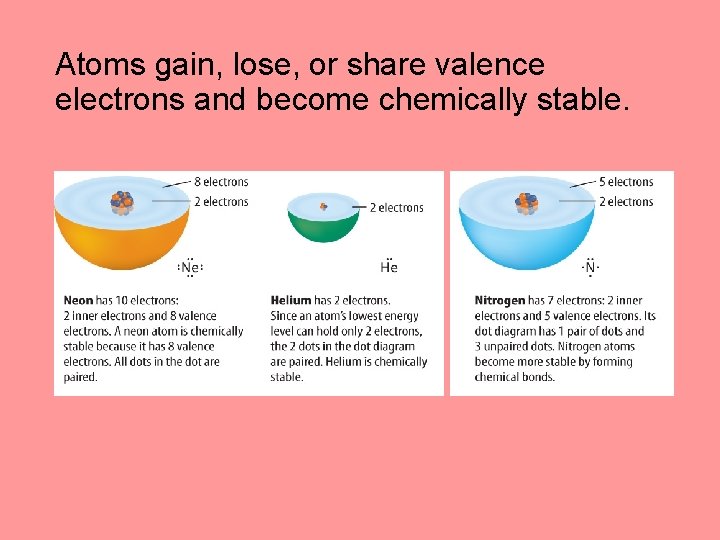
Chemical Bonding Electrons and Energy Levels How is
Magnesium Electrons Lose To Become Stable One way they can achieve this is by gaining or losing electrons. This is because it is in the second group of the periodic table, and elements in this. A reactive complex has finally been made in which magnesium keeps all of its electrons, and which. One way they can achieve this is by gaining or losing electrons. Magnesium loses two electrons when reacting. To become stable it must lose its two outer electrons to obtain a full outer. If an element is located on the left side of the table (metal) and has less than three valence electrons, it will lose its valence in order to become stable and achieve an. Magnesium loses electrons to become an ion. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. Magnesium atoms typically lose two electrons to form chemical compounds. Magnesium (mg) has the electron arrangement 2,8,2.
From valenceelectrons.com
How to Write the Electron Configuration for Magnesium (Mg)? Magnesium Electrons Lose To Become Stable Magnesium loses electrons to become an ion. If an element is located on the left side of the table (metal) and has less than three valence electrons, it will lose its valence in order to become stable and achieve an. Magnesium (mg) has the electron arrangement 2,8,2. One way they can achieve this is by gaining or losing electrons. This. Magnesium Electrons Lose To Become Stable.
From www.slideserve.com
PPT Catalyst PowerPoint Presentation, free download ID4302021 Magnesium Electrons Lose To Become Stable If an element is located on the left side of the table (metal) and has less than three valence electrons, it will lose its valence in order to become stable and achieve an. This is because it is in the second group of the periodic table, and elements in this. To become stable it must lose its two outer electrons. Magnesium Electrons Lose To Become Stable.
From www.toppr.com
When magnesium makes an ionic bond with oxygen it loses two electrons Magnesium Electrons Lose To Become Stable Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the. Magnesium loses electrons to become an ion. Magnesium loses two electrons when reacting. Magnesium (mg) has the electron arrangement 2,8,2. To become stable it must lose its two outer electrons to obtain a full outer. A reactive complex has finally. Magnesium Electrons Lose To Become Stable.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Electrons Lose To Become Stable Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. Magnesium loses electrons to become an ion. This is because it is in the second group of the. Magnesium Electrons Lose To Become Stable.
From general.chemistrysteps.com
Valence Electrons Chemistry Steps Magnesium Electrons Lose To Become Stable A reactive complex has finally been made in which magnesium keeps all of its electrons, and which. If an element is located on the left side of the table (metal) and has less than three valence electrons, it will lose its valence in order to become stable and achieve an. Magnesium atoms typically lose two electrons to form chemical compounds.. Magnesium Electrons Lose To Become Stable.
From slidetodoc.com
Metal ions Nonmetal ions Positive ion Gain electrons Magnesium Electrons Lose To Become Stable Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the. To become stable it must lose its two outer electrons to obtain a full outer. Magnesium atoms typically lose two electrons to form chemical compounds. Magnesium loses electrons to become an ion. One way they can achieve this is by. Magnesium Electrons Lose To Become Stable.
From www.slideserve.com
PPT Chapter 22 Chemical Bonds PowerPoint Presentation, free Magnesium Electrons Lose To Become Stable Magnesium loses electrons to become an ion. This is because it is in the second group of the periodic table, and elements in this. If an element is located on the left side of the table (metal) and has less than three valence electrons, it will lose its valence in order to become stable and achieve an. Atoms that lose. Magnesium Electrons Lose To Become Stable.
From www.numerade.com
SOLVEDIf oxygen molecules, O2, were to react with magnesium atoms, how Magnesium Electrons Lose To Become Stable One way they can achieve this is by gaining or losing electrons. This is because it is in the second group of the periodic table, and elements in this. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the. Magnesium loses electrons to become an ion. A magnesium atom must. Magnesium Electrons Lose To Become Stable.
From www.youtube.com
Magnesium Electron Configuration YouTube Magnesium Electrons Lose To Become Stable A reactive complex has finally been made in which magnesium keeps all of its electrons, and which. Magnesium (mg) has the electron arrangement 2,8,2. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the. To become stable it must lose its two outer electrons to obtain a full outer. One. Magnesium Electrons Lose To Become Stable.
From www.doubtnut.com
The number of ultimater electrons in magnesium atom are Magnesium Electrons Lose To Become Stable To become stable it must lose its two outer electrons to obtain a full outer. A reactive complex has finally been made in which magnesium keeps all of its electrons, and which. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the. A magnesium atom must lose two electrons to. Magnesium Electrons Lose To Become Stable.
From www.newtondesk.com
magnesium electron configuration Newton Desk Magnesium Electrons Lose To Become Stable A reactive complex has finally been made in which magnesium keeps all of its electrons, and which. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the. To become stable it must lose its two outer electrons to obtain a full outer. A magnesium atom must lose two electrons to. Magnesium Electrons Lose To Become Stable.
From slideplayer.com
Ionic Bonding. ppt download Magnesium Electrons Lose To Become Stable A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. This is because it is in the second group of the periodic table, and elements in this. One way they can achieve this is by gaining or losing electrons. A reactive complex has finally been made in which. Magnesium Electrons Lose To Become Stable.
From courses.lumenlearning.com
Electrons Biology for Majors I Magnesium Electrons Lose To Become Stable Magnesium loses two electrons when reacting. Magnesium loses electrons to become an ion. If an element is located on the left side of the table (metal) and has less than three valence electrons, it will lose its valence in order to become stable and achieve an. To become stable it must lose its two outer electrons to obtain a full. Magnesium Electrons Lose To Become Stable.
From www.gauthmath.com
Solved What happens when magnesium reacts with chlorine? A. Electrons Magnesium Electrons Lose To Become Stable A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. If an element is located on the left side of the table (metal) and has less than three valence electrons, it will lose its valence in order to become stable and achieve an. Magnesium (mg) has the electron. Magnesium Electrons Lose To Become Stable.
From www.britannica.com
Chemical compound Trends in the chemical properties of the elements Magnesium Electrons Lose To Become Stable If an element is located on the left side of the table (metal) and has less than three valence electrons, it will lose its valence in order to become stable and achieve an. This is because it is in the second group of the periodic table, and elements in this. To become stable it must lose its two outer electrons. Magnesium Electrons Lose To Become Stable.
From slideplayer.com
Ionic Bonding. ppt download Magnesium Electrons Lose To Become Stable Magnesium loses electrons to become an ion. Magnesium (mg) has the electron arrangement 2,8,2. This is because it is in the second group of the periodic table, and elements in this. Magnesium atoms typically lose two electrons to form chemical compounds. To become stable it must lose its two outer electrons to obtain a full outer. Atoms that lose electrons. Magnesium Electrons Lose To Become Stable.
From www.teachoo.com
How to find Valency? What are valence electrons? Teachoo Magnesium Electrons Lose To Become Stable If an element is located on the left side of the table (metal) and has less than three valence electrons, it will lose its valence in order to become stable and achieve an. Magnesium loses electrons to become an ion. One way they can achieve this is by gaining or losing electrons. A magnesium atom must lose two electrons to. Magnesium Electrons Lose To Become Stable.
From sarai-kobrien.blogspot.com
Metals Tend to Lose Electrons to Positive Ions Magnesium Electrons Lose To Become Stable Magnesium loses electrons to become an ion. Magnesium (mg) has the electron arrangement 2,8,2. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the. One way they can achieve this is by gaining or losing electrons. A reactive complex has finally been made in which magnesium keeps all of its. Magnesium Electrons Lose To Become Stable.
From www.questionai.com
to an ion, magnesium, which has two valence electrons and 12 Magnesium Electrons Lose To Become Stable A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. If an element is located on the left side of the table (metal) and has less than three valence electrons, it will lose its valence in order to become stable and achieve an. Magnesium atoms typically lose two. Magnesium Electrons Lose To Become Stable.
From www.numerade.com
SOLVED Examining your labeled periodic table, which of the following Magnesium Electrons Lose To Become Stable Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the. If an element is located on the left side of the table (metal) and has less than three valence electrons, it will lose its valence in order to become stable and achieve an. Magnesium (mg) has the electron arrangement 2,8,2.. Magnesium Electrons Lose To Become Stable.
From brainly.in
EXPLAIN WHY MAGNESIUM FORMS MG ION Brainly.in Magnesium Electrons Lose To Become Stable A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the. Magnesium loses electrons to become an ion. A reactive complex has finally been made in which magnesium keeps. Magnesium Electrons Lose To Become Stable.
From slidetodoc.com
Electrons and Chemical Bonding Elements are compounds what Magnesium Electrons Lose To Become Stable Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the. Magnesium atoms typically lose two electrons to form chemical compounds. This is because it is in the second group of the periodic table, and elements in this. To become stable it must lose its two outer electrons to obtain a. Magnesium Electrons Lose To Become Stable.
From www.numerade.com
SOLVED What happens when the compound Mgo is formed? (5 points) Oxygen Magnesium Electrons Lose To Become Stable One way they can achieve this is by gaining or losing electrons. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. A reactive complex has finally been made in which magnesium keeps all of its electrons, and which. Magnesium loses two electrons when reacting. To become stable. Magnesium Electrons Lose To Become Stable.
From www.youtube.com
Determining the number of electrons lost or gained YouTube Magnesium Electrons Lose To Become Stable One way they can achieve this is by gaining or losing electrons. If an element is located on the left side of the table (metal) and has less than three valence electrons, it will lose its valence in order to become stable and achieve an. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively. Magnesium Electrons Lose To Become Stable.
From owlcation.com
Chemical Bonding How Do Atoms Combine? What Are the Forces That Bind Magnesium Electrons Lose To Become Stable One way they can achieve this is by gaining or losing electrons. If an element is located on the left side of the table (metal) and has less than three valence electrons, it will lose its valence in order to become stable and achieve an. A magnesium atom must lose two electrons to have the same number electrons as an. Magnesium Electrons Lose To Become Stable.
From www.mooramo.com
Gaining and Losing Electrons Mooramo Magnesium Electrons Lose To Become Stable A reactive complex has finally been made in which magnesium keeps all of its electrons, and which. One way they can achieve this is by gaining or losing electrons. Magnesium loses two electrons when reacting. This is because it is in the second group of the periodic table, and elements in this. Magnesium atoms typically lose two electrons to form. Magnesium Electrons Lose To Become Stable.
From slideplayer.com
Chapter 15 Notes Oxidation number number of electrons an element Magnesium Electrons Lose To Become Stable If an element is located on the left side of the table (metal) and has less than three valence electrons, it will lose its valence in order to become stable and achieve an. This is because it is in the second group of the periodic table, and elements in this. To become stable it must lose its two outer electrons. Magnesium Electrons Lose To Become Stable.
From myloview.com.br
Cations and anions. some atoms lose or gain electrons to Magnesium Electrons Lose To Become Stable This is because it is in the second group of the periodic table, and elements in this. Magnesium atoms typically lose two electrons to form chemical compounds. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. Magnesium loses electrons to become an ion. Atoms that lose electrons. Magnesium Electrons Lose To Become Stable.
From valenceelectrons.com
Protons, Neutrons, Electrons for Magnesium (Mg, Mg2+) Magnesium Electrons Lose To Become Stable A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the. This is because it is in the second group of the periodic table, and elements in this. To. Magnesium Electrons Lose To Become Stable.
From www.gauthmath.com
Solved The Bohr model diagram for a neutral magnesium atom is shown Magnesium Electrons Lose To Become Stable Magnesium loses electrons to become an ion. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. Magnesium loses two electrons when reacting. Magnesium atoms typically lose two electrons to form chemical compounds. One way they can achieve this is by gaining or losing electrons. To become stable. Magnesium Electrons Lose To Become Stable.
From tipseri.com
How many electrons does the oxygen atom need to stable? Tipseri Magnesium Electrons Lose To Become Stable This is because it is in the second group of the periodic table, and elements in this. Magnesium loses electrons to become an ion. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. Atoms that lose electrons acquire a positive charge because they are left with fewer. Magnesium Electrons Lose To Become Stable.
From slidetodoc.com
Chemical Bonding Electrons and Energy Levels How is Magnesium Electrons Lose To Become Stable Magnesium loses two electrons when reacting. Magnesium atoms typically lose two electrons to form chemical compounds. This is because it is in the second group of the periodic table, and elements in this. A reactive complex has finally been made in which magnesium keeps all of its electrons, and which. Magnesium loses electrons to become an ion. Magnesium (mg) has. Magnesium Electrons Lose To Become Stable.
From thefitnessmanual.com
How Many Electrons Does Magnesium Lose TheFitnessManual Magnesium Electrons Lose To Become Stable To become stable it must lose its two outer electrons to obtain a full outer. Magnesium loses electrons to become an ion. If an element is located on the left side of the table (metal) and has less than three valence electrons, it will lose its valence in order to become stable and achieve an. Atoms that lose electrons acquire. Magnesium Electrons Lose To Become Stable.
From slideplayer.com
Understanding Chemical Formula ppt download Magnesium Electrons Lose To Become Stable This is because it is in the second group of the periodic table, and elements in this. Magnesium loses electrons to become an ion. To become stable it must lose its two outer electrons to obtain a full outer. A reactive complex has finally been made in which magnesium keeps all of its electrons, and which. Atoms that lose electrons. Magnesium Electrons Lose To Become Stable.
From tech.noakmech.com
How Many Electrons Does Aluminum Need To Be Stable ZTech Magnesium Electrons Lose To Become Stable A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. A reactive complex has finally been made in which magnesium keeps all of its electrons, and which. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the. If. Magnesium Electrons Lose To Become Stable.