What Is Reduction In Organic Chemistry . See examples of common reducing agents, such as lialh4, nabh4,. Learn how to identify and rank organic reactions as oxidations, reductions, or neither based on the change in oxidation state of carbon. It describes common reduction and oxidation in organic chemistry including typical reducing and oxidizing reagents, their usages,. See charts and examples of. See examples of common functional groups and their oxidation levels, and how to use oxidation state formalism to track electrons in organic molecules. We more often use the mnemonic you have. Learn how to identify oxidation and reduction processes in organic reactions by using a simplified definition based on bond types and electronegativity. In organic chemistry, we rarely calculate out the actual oxidation state of carbon. Learn about different types of reductions in organic chemistry, such as hydride transfer, carbonyl, halide and nitrile reductions. A reducing agent, also known as a reductant, is a substance that has the ability to donate electrons to. We’ve pointed out on several occasions that some of the reactions. 10.8 • oxidation and reduction in organic chemistry.
from www.masterorganicchemistry.com
We’ve pointed out on several occasions that some of the reactions. See examples of common functional groups and their oxidation levels, and how to use oxidation state formalism to track electrons in organic molecules. A reducing agent, also known as a reductant, is a substance that has the ability to donate electrons to. Learn about different types of reductions in organic chemistry, such as hydride transfer, carbonyl, halide and nitrile reductions. It describes common reduction and oxidation in organic chemistry including typical reducing and oxidizing reagents, their usages,. See charts and examples of. 10.8 • oxidation and reduction in organic chemistry. In organic chemistry, we rarely calculate out the actual oxidation state of carbon. Learn how to identify and rank organic reactions as oxidations, reductions, or neither based on the change in oxidation state of carbon. We more often use the mnemonic you have.
Sodium Borohydride (NaBH4) As A Reagent In Organic Chemistry
What Is Reduction In Organic Chemistry We’ve pointed out on several occasions that some of the reactions. A reducing agent, also known as a reductant, is a substance that has the ability to donate electrons to. See charts and examples of. It describes common reduction and oxidation in organic chemistry including typical reducing and oxidizing reagents, their usages,. We more often use the mnemonic you have. 10.8 • oxidation and reduction in organic chemistry. In organic chemistry, we rarely calculate out the actual oxidation state of carbon. Learn how to identify oxidation and reduction processes in organic reactions by using a simplified definition based on bond types and electronegativity. See examples of common functional groups and their oxidation levels, and how to use oxidation state formalism to track electrons in organic molecules. See examples of common reducing agents, such as lialh4, nabh4,. We’ve pointed out on several occasions that some of the reactions. Learn how to identify and rank organic reactions as oxidations, reductions, or neither based on the change in oxidation state of carbon. Learn about different types of reductions in organic chemistry, such as hydride transfer, carbonyl, halide and nitrile reductions.
From www.pinterest.com
Oxidation and Reduction Reactions Study Guide Cheat Sheet Teaching What Is Reduction In Organic Chemistry We more often use the mnemonic you have. Learn how to identify oxidation and reduction processes in organic reactions by using a simplified definition based on bond types and electronegativity. See examples of common reducing agents, such as lialh4, nabh4,. In organic chemistry, we rarely calculate out the actual oxidation state of carbon. See charts and examples of. See examples. What Is Reduction In Organic Chemistry.
From www.chegg.com
Solved Part 1 Oxidation/Reduction in Organic Chemistry What Is Reduction In Organic Chemistry We more often use the mnemonic you have. Learn how to identify and rank organic reactions as oxidations, reductions, or neither based on the change in oxidation state of carbon. See examples of common reducing agents, such as lialh4, nabh4,. See charts and examples of. We’ve pointed out on several occasions that some of the reactions. Learn how to identify. What Is Reduction In Organic Chemistry.
From www.chemistrylearner.com
Redox (OxidationReduction) Reaction Definition & Examples What Is Reduction In Organic Chemistry Learn how to identify oxidation and reduction processes in organic reactions by using a simplified definition based on bond types and electronegativity. See examples of common reducing agents, such as lialh4, nabh4,. We more often use the mnemonic you have. 10.8 • oxidation and reduction in organic chemistry. It describes common reduction and oxidation in organic chemistry including typical reducing. What Is Reduction In Organic Chemistry.
From www.masterorganicchemistry.com
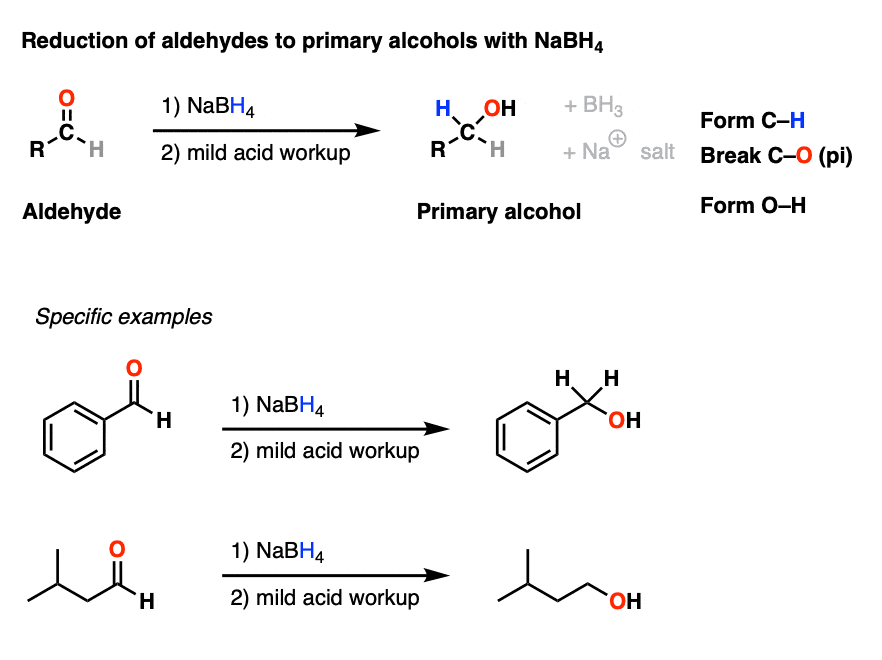
Addition of NaBH4 to aldehydes to give primary alcohols Master What Is Reduction In Organic Chemistry It describes common reduction and oxidation in organic chemistry including typical reducing and oxidizing reagents, their usages,. Learn how to identify and rank organic reactions as oxidations, reductions, or neither based on the change in oxidation state of carbon. 10.8 • oxidation and reduction in organic chemistry. See examples of common functional groups and their oxidation levels, and how to. What Is Reduction In Organic Chemistry.
From www.organicchemistrytutor.com
Reactions of Aldehydes and Ketones — Organic Chemistry Tutor What Is Reduction In Organic Chemistry 10.8 • oxidation and reduction in organic chemistry. In organic chemistry, we rarely calculate out the actual oxidation state of carbon. A reducing agent, also known as a reductant, is a substance that has the ability to donate electrons to. See examples of common functional groups and their oxidation levels, and how to use oxidation state formalism to track electrons. What Is Reduction In Organic Chemistry.
From www.vrogue.co
Oxidation And Reduction Reactions Chemistry Libretext vrogue.co What Is Reduction In Organic Chemistry See examples of common functional groups and their oxidation levels, and how to use oxidation state formalism to track electrons in organic molecules. Learn how to identify and rank organic reactions as oxidations, reductions, or neither based on the change in oxidation state of carbon. We’ve pointed out on several occasions that some of the reactions. See charts and examples. What Is Reduction In Organic Chemistry.
From www.masterorganicchemistry.com
Imines Properties, Formation, Reactions, and Mechanisms Master What Is Reduction In Organic Chemistry Learn about different types of reductions in organic chemistry, such as hydride transfer, carbonyl, halide and nitrile reductions. We’ve pointed out on several occasions that some of the reactions. See examples of common reducing agents, such as lialh4, nabh4,. Learn how to identify oxidation and reduction processes in organic reactions by using a simplified definition based on bond types and. What Is Reduction In Organic Chemistry.
From www.youtube.com
Oxidation and Reduction Reactions Basic Introduction YouTube What Is Reduction In Organic Chemistry A reducing agent, also known as a reductant, is a substance that has the ability to donate electrons to. Learn about different types of reductions in organic chemistry, such as hydride transfer, carbonyl, halide and nitrile reductions. See examples of common functional groups and their oxidation levels, and how to use oxidation state formalism to track electrons in organic molecules.. What Is Reduction In Organic Chemistry.
From www.masterorganicchemistry.com
Hydroboration of Alkenes Master Organic Chemistry What Is Reduction In Organic Chemistry We’ve pointed out on several occasions that some of the reactions. In organic chemistry, we rarely calculate out the actual oxidation state of carbon. See charts and examples of. It describes common reduction and oxidation in organic chemistry including typical reducing and oxidizing reagents, their usages,. See examples of common reducing agents, such as lialh4, nabh4,. Learn about different types. What Is Reduction In Organic Chemistry.
From www.organicchemistrytutor.com
Synthesis and Reactions of Amines — Organic Chemistry Tutor What Is Reduction In Organic Chemistry Learn how to identify and rank organic reactions as oxidations, reductions, or neither based on the change in oxidation state of carbon. Learn how to identify oxidation and reduction processes in organic reactions by using a simplified definition based on bond types and electronegativity. Learn about different types of reductions in organic chemistry, such as hydride transfer, carbonyl, halide and. What Is Reduction In Organic Chemistry.
From www.slideserve.com
PPT Introduction to Organic Chemistry PowerPoint Presentation, free What Is Reduction In Organic Chemistry See examples of common reducing agents, such as lialh4, nabh4,. In organic chemistry, we rarely calculate out the actual oxidation state of carbon. 10.8 • oxidation and reduction in organic chemistry. See examples of common functional groups and their oxidation levels, and how to use oxidation state formalism to track electrons in organic molecules. We’ve pointed out on several occasions. What Is Reduction In Organic Chemistry.
From ar.inspiredpencil.com
Reduction Reaction Organic Chemistry What Is Reduction In Organic Chemistry See charts and examples of. See examples of common functional groups and their oxidation levels, and how to use oxidation state formalism to track electrons in organic molecules. It describes common reduction and oxidation in organic chemistry including typical reducing and oxidizing reagents, their usages,. 10.8 • oxidation and reduction in organic chemistry. See examples of common reducing agents, such. What Is Reduction In Organic Chemistry.
From ar.inspiredpencil.com
Reduction Reaction Organic Chemistry What Is Reduction In Organic Chemistry See examples of common functional groups and their oxidation levels, and how to use oxidation state formalism to track electrons in organic molecules. We’ve pointed out on several occasions that some of the reactions. In organic chemistry, we rarely calculate out the actual oxidation state of carbon. Learn about different types of reductions in organic chemistry, such as hydride transfer,. What Is Reduction In Organic Chemistry.
From www.pinterest.com
Oxidation Reduction (Redox) Reactions Balancing Redox Reactions What Is Reduction In Organic Chemistry It describes common reduction and oxidation in organic chemistry including typical reducing and oxidizing reagents, their usages,. 10.8 • oxidation and reduction in organic chemistry. In organic chemistry, we rarely calculate out the actual oxidation state of carbon. Learn about different types of reductions in organic chemistry, such as hydride transfer, carbonyl, halide and nitrile reductions. See examples of common. What Is Reduction In Organic Chemistry.
From www.chemistrysteps.com
LiAlH4 and NaBH4 Carbonyl Reduction Mechanism Chemistry Steps What Is Reduction In Organic Chemistry Learn how to identify oxidation and reduction processes in organic reactions by using a simplified definition based on bond types and electronegativity. It describes common reduction and oxidation in organic chemistry including typical reducing and oxidizing reagents, their usages,. Learn how to identify and rank organic reactions as oxidations, reductions, or neither based on the change in oxidation state of. What Is Reduction In Organic Chemistry.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Redox reaction What Is Reduction In Organic Chemistry See charts and examples of. It describes common reduction and oxidation in organic chemistry including typical reducing and oxidizing reagents, their usages,. 10.8 • oxidation and reduction in organic chemistry. Learn how to identify oxidation and reduction processes in organic reactions by using a simplified definition based on bond types and electronegativity. See examples of common reducing agents, such as. What Is Reduction In Organic Chemistry.
From www.masterorganicchemistry.com
Oxidation and Reduction in Organic Chemistry Master Organic Chemistry What Is Reduction In Organic Chemistry It describes common reduction and oxidation in organic chemistry including typical reducing and oxidizing reagents, their usages,. We more often use the mnemonic you have. A reducing agent, also known as a reductant, is a substance that has the ability to donate electrons to. 10.8 • oxidation and reduction in organic chemistry. Learn how to identify oxidation and reduction processes. What Is Reduction In Organic Chemistry.
From www.pinterest.com
Sodium Borohydride NaBH4 Reduction Reaction Mechanism Organic What Is Reduction In Organic Chemistry Learn how to identify and rank organic reactions as oxidations, reductions, or neither based on the change in oxidation state of carbon. See examples of common functional groups and their oxidation levels, and how to use oxidation state formalism to track electrons in organic molecules. It describes common reduction and oxidation in organic chemistry including typical reducing and oxidizing reagents,. What Is Reduction In Organic Chemistry.
From leah4sci.com
Oxidation and Reduction Reactions in MCAT General Chemistry What Is Reduction In Organic Chemistry See charts and examples of. Learn how to identify oxidation and reduction processes in organic reactions by using a simplified definition based on bond types and electronegativity. See examples of common reducing agents, such as lialh4, nabh4,. We’ve pointed out on several occasions that some of the reactions. We more often use the mnemonic you have. Learn about different types. What Is Reduction In Organic Chemistry.
From www.youtube.com
Intro to Oxidation and Reduction Reactions in Organic Chemistry YouTube What Is Reduction In Organic Chemistry See charts and examples of. In organic chemistry, we rarely calculate out the actual oxidation state of carbon. It describes common reduction and oxidation in organic chemistry including typical reducing and oxidizing reagents, their usages,. Learn how to identify and rank organic reactions as oxidations, reductions, or neither based on the change in oxidation state of carbon. Learn how to. What Is Reduction In Organic Chemistry.
From ncstate.pressbooks.pub
10.8 Oxidation and Reduction in Organic Chemistry Organic Chemistry What Is Reduction In Organic Chemistry It describes common reduction and oxidation in organic chemistry including typical reducing and oxidizing reagents, their usages,. 10.8 • oxidation and reduction in organic chemistry. Learn how to identify and rank organic reactions as oxidations, reductions, or neither based on the change in oxidation state of carbon. See examples of common reducing agents, such as lialh4, nabh4,. A reducing agent,. What Is Reduction In Organic Chemistry.
From ar.inspiredpencil.com
Reduction Reaction Organic Chemistry What Is Reduction In Organic Chemistry See examples of common reducing agents, such as lialh4, nabh4,. See examples of common functional groups and their oxidation levels, and how to use oxidation state formalism to track electrons in organic molecules. Learn about different types of reductions in organic chemistry, such as hydride transfer, carbonyl, halide and nitrile reductions. We’ve pointed out on several occasions that some of. What Is Reduction In Organic Chemistry.
From 2012books.lardbucket.org
Common Classes of Organic Reactions What Is Reduction In Organic Chemistry See examples of common functional groups and their oxidation levels, and how to use oxidation state formalism to track electrons in organic molecules. We more often use the mnemonic you have. In organic chemistry, we rarely calculate out the actual oxidation state of carbon. It describes common reduction and oxidation in organic chemistry including typical reducing and oxidizing reagents, their. What Is Reduction In Organic Chemistry.
From www.masterorganicchemistry.com
Sodium Borohydride (NaBH4) As A Reagent In Organic Chemistry What Is Reduction In Organic Chemistry See examples of common reducing agents, such as lialh4, nabh4,. See charts and examples of. A reducing agent, also known as a reductant, is a substance that has the ability to donate electrons to. Learn how to identify and rank organic reactions as oxidations, reductions, or neither based on the change in oxidation state of carbon. We more often use. What Is Reduction In Organic Chemistry.
From www.masterorganicchemistry.com
Fischer Esterification Carboxylic Acid to Ester Under Acidic What Is Reduction In Organic Chemistry Learn how to identify oxidation and reduction processes in organic reactions by using a simplified definition based on bond types and electronegativity. See charts and examples of. A reducing agent, also known as a reductant, is a substance that has the ability to donate electrons to. See examples of common functional groups and their oxidation levels, and how to use. What Is Reduction In Organic Chemistry.
From leah4sci.com
Oxidation and Reduction Reactions in Organic Chemistry What Is Reduction In Organic Chemistry We’ve pointed out on several occasions that some of the reactions. A reducing agent, also known as a reductant, is a substance that has the ability to donate electrons to. It describes common reduction and oxidation in organic chemistry including typical reducing and oxidizing reagents, their usages,. Learn how to identify oxidation and reduction processes in organic reactions by using. What Is Reduction In Organic Chemistry.
From www.slideserve.com
PPT Chapter 10 Organohalides PowerPoint Presentation, free download What Is Reduction In Organic Chemistry See examples of common reducing agents, such as lialh4, nabh4,. We more often use the mnemonic you have. It describes common reduction and oxidation in organic chemistry including typical reducing and oxidizing reagents, their usages,. We’ve pointed out on several occasions that some of the reactions. Learn about different types of reductions in organic chemistry, such as hydride transfer, carbonyl,. What Is Reduction In Organic Chemistry.
From www.youtube.com
REDUCTION REACTIONSIIONE SHOT REVISION TO REACTIONS IN ORGANIC What Is Reduction In Organic Chemistry A reducing agent, also known as a reductant, is a substance that has the ability to donate electrons to. Learn about different types of reductions in organic chemistry, such as hydride transfer, carbonyl, halide and nitrile reductions. Learn how to identify oxidation and reduction processes in organic reactions by using a simplified definition based on bond types and electronegativity. See. What Is Reduction In Organic Chemistry.
From www.pinterest.com
Reduction Reactions and Common Reducing Agents Teaching chemistry What Is Reduction In Organic Chemistry We’ve pointed out on several occasions that some of the reactions. See examples of common reducing agents, such as lialh4, nabh4,. In organic chemistry, we rarely calculate out the actual oxidation state of carbon. Learn about different types of reductions in organic chemistry, such as hydride transfer, carbonyl, halide and nitrile reductions. Learn how to identify oxidation and reduction processes. What Is Reduction In Organic Chemistry.
From ar.inspiredpencil.com
Reduction Reaction Organic Chemistry What Is Reduction In Organic Chemistry It describes common reduction and oxidation in organic chemistry including typical reducing and oxidizing reagents, their usages,. A reducing agent, also known as a reductant, is a substance that has the ability to donate electrons to. See examples of common reducing agents, such as lialh4, nabh4,. In organic chemistry, we rarely calculate out the actual oxidation state of carbon. We’ve. What Is Reduction In Organic Chemistry.
From in.pinterest.com
Difference Between Oxidation and Reduction Definition, Mechanism What Is Reduction In Organic Chemistry It describes common reduction and oxidation in organic chemistry including typical reducing and oxidizing reagents, their usages,. A reducing agent, also known as a reductant, is a substance that has the ability to donate electrons to. 10.8 • oxidation and reduction in organic chemistry. Learn about different types of reductions in organic chemistry, such as hydride transfer, carbonyl, halide and. What Is Reduction In Organic Chemistry.
From organicpotroga.blogspot.com
Organic Basics Of Organic Chemistry What Is Reduction In Organic Chemistry In organic chemistry, we rarely calculate out the actual oxidation state of carbon. 10.8 • oxidation and reduction in organic chemistry. We’ve pointed out on several occasions that some of the reactions. It describes common reduction and oxidation in organic chemistry including typical reducing and oxidizing reagents, their usages,. Learn about different types of reductions in organic chemistry, such as. What Is Reduction In Organic Chemistry.
From www.masterorganicchemistry.com
Alcohol Oxidation "Strong" & "Weak" Oxidants Master Organic Chemistry What Is Reduction In Organic Chemistry Learn about different types of reductions in organic chemistry, such as hydride transfer, carbonyl, halide and nitrile reductions. We’ve pointed out on several occasions that some of the reactions. See examples of common reducing agents, such as lialh4, nabh4,. In organic chemistry, we rarely calculate out the actual oxidation state of carbon. We more often use the mnemonic you have.. What Is Reduction In Organic Chemistry.
From www.masterorganicchemistry.com
Alcohol Oxidation "Strong" & "Weak" Oxidants Master Organic Chemistry What Is Reduction In Organic Chemistry See examples of common functional groups and their oxidation levels, and how to use oxidation state formalism to track electrons in organic molecules. Learn how to identify and rank organic reactions as oxidations, reductions, or neither based on the change in oxidation state of carbon. In organic chemistry, we rarely calculate out the actual oxidation state of carbon. See examples. What Is Reduction In Organic Chemistry.
From openpress.usask.ca
7.4. Oxidation States Introduction to Organic Chemistry What Is Reduction In Organic Chemistry In organic chemistry, we rarely calculate out the actual oxidation state of carbon. It describes common reduction and oxidation in organic chemistry including typical reducing and oxidizing reagents, their usages,. Learn how to identify oxidation and reduction processes in organic reactions by using a simplified definition based on bond types and electronegativity. See charts and examples of. 10.8 • oxidation. What Is Reduction In Organic Chemistry.