Joint Commission Syringe Labeling Requirements . Requirements for labeling medications in the perioperative setting (joint commission, 2020). Drug, strength, date, time drawn and the initials of the person who prepared the syringe. Food and drug administration (fda), manufacturers of certain medical devices and products must include. Syringes require 5 labeling elements: Home joint commission accreditation ambulatory national patient safety goals (npsg) standards. All solutions on the sterile field, including medications, saline, and/or water, should be labeled with name, strength, dosage, and. As stated in the elements of performance for. The only requirements for labeling include the name, strength, amount, and expiration date that are already on the manufacturer's. National patient safety goal (npsg) 03.04.01, to improve the safety of using medications, was first.
from syringepumppro.com
Requirements for labeling medications in the perioperative setting (joint commission, 2020). All solutions on the sterile field, including medications, saline, and/or water, should be labeled with name, strength, dosage, and. Syringes require 5 labeling elements: National patient safety goal (npsg) 03.04.01, to improve the safety of using medications, was first. Food and drug administration (fda), manufacturers of certain medical devices and products must include. As stated in the elements of performance for. Home joint commission accreditation ambulatory national patient safety goals (npsg) standards. Drug, strength, date, time drawn and the initials of the person who prepared the syringe. The only requirements for labeling include the name, strength, amount, and expiration date that are already on the manufacturer's.
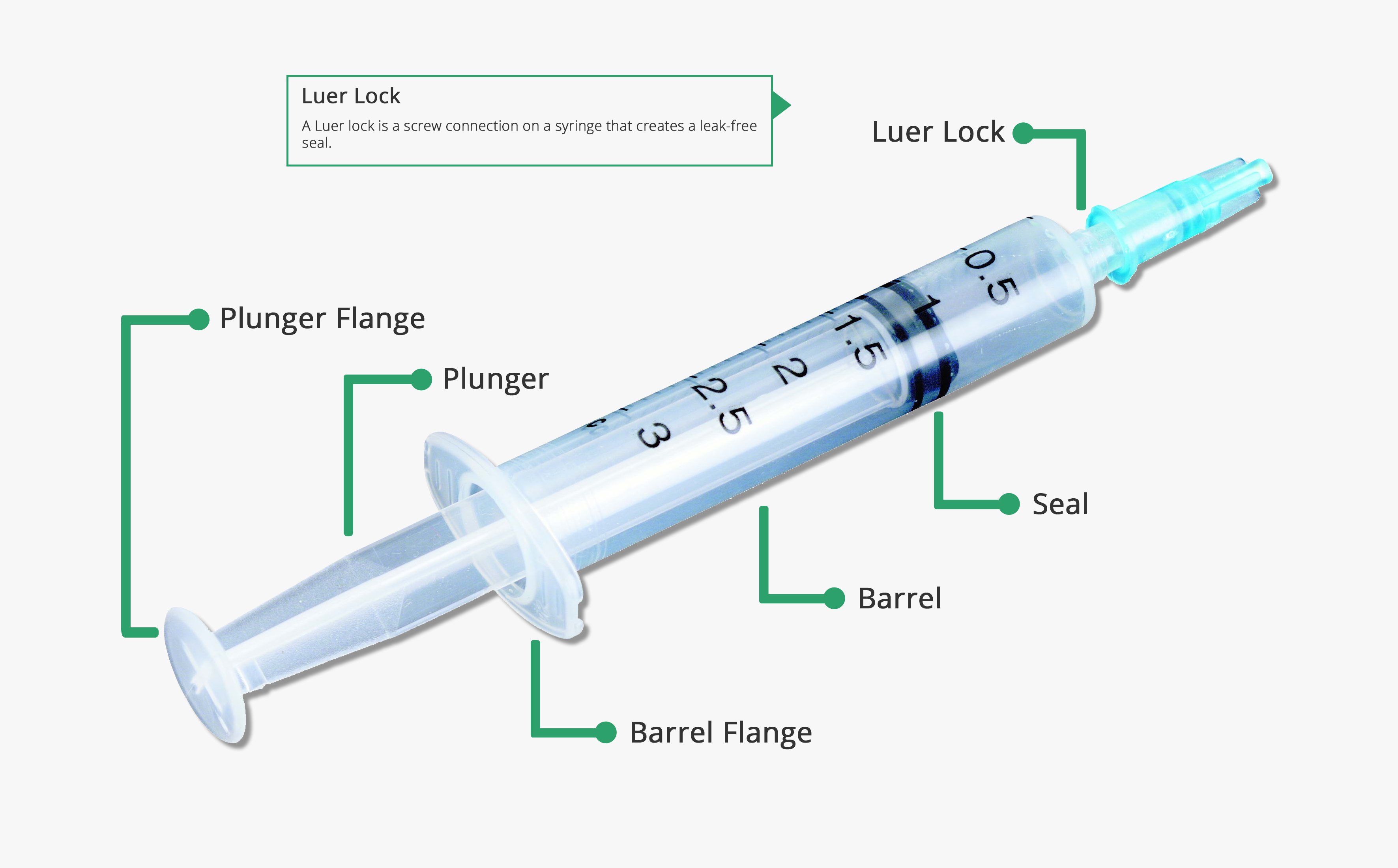
Parts Of A Syringe SyringePumpPro
Joint Commission Syringe Labeling Requirements Home joint commission accreditation ambulatory national patient safety goals (npsg) standards. Home joint commission accreditation ambulatory national patient safety goals (npsg) standards. Food and drug administration (fda), manufacturers of certain medical devices and products must include. As stated in the elements of performance for. Requirements for labeling medications in the perioperative setting (joint commission, 2020). The only requirements for labeling include the name, strength, amount, and expiration date that are already on the manufacturer's. All solutions on the sterile field, including medications, saline, and/or water, should be labeled with name, strength, dosage, and. Syringes require 5 labeling elements: National patient safety goal (npsg) 03.04.01, to improve the safety of using medications, was first. Drug, strength, date, time drawn and the initials of the person who prepared the syringe.
From www.lss-dk.com
Syringe labelling machines Automatic labelling LSS Joint Commission Syringe Labeling Requirements Syringes require 5 labeling elements: Drug, strength, date, time drawn and the initials of the person who prepared the syringe. National patient safety goal (npsg) 03.04.01, to improve the safety of using medications, was first. Food and drug administration (fda), manufacturers of certain medical devices and products must include. All solutions on the sterile field, including medications, saline, and/or water,. Joint Commission Syringe Labeling Requirements.
From www.vrogue.co
Parts Of A Syringe Labeled vrogue.co Joint Commission Syringe Labeling Requirements Requirements for labeling medications in the perioperative setting (joint commission, 2020). Drug, strength, date, time drawn and the initials of the person who prepared the syringe. Food and drug administration (fda), manufacturers of certain medical devices and products must include. As stated in the elements of performance for. Home joint commission accreditation ambulatory national patient safety goals (npsg) standards. The. Joint Commission Syringe Labeling Requirements.
From dandelionsandthings.blogspot.com
34 Label Parts Of A Syringe Label Design Ideas 2020 Joint Commission Syringe Labeling Requirements Drug, strength, date, time drawn and the initials of the person who prepared the syringe. Food and drug administration (fda), manufacturers of certain medical devices and products must include. National patient safety goal (npsg) 03.04.01, to improve the safety of using medications, was first. As stated in the elements of performance for. All solutions on the sterile field, including medications,. Joint Commission Syringe Labeling Requirements.
From www.labeltemplate.net
5 The Complete Guide to Syringe Labels & How They are Keeping You Protected label template Joint Commission Syringe Labeling Requirements Requirements for labeling medications in the perioperative setting (joint commission, 2020). Food and drug administration (fda), manufacturers of certain medical devices and products must include. Home joint commission accreditation ambulatory national patient safety goals (npsg) standards. The only requirements for labeling include the name, strength, amount, and expiration date that are already on the manufacturer's. As stated in the elements. Joint Commission Syringe Labeling Requirements.
From gioydipcr.blob.core.windows.net
Joint Commission Labeling Syringes at Alice Cobb blog Joint Commission Syringe Labeling Requirements Home joint commission accreditation ambulatory national patient safety goals (npsg) standards. The only requirements for labeling include the name, strength, amount, and expiration date that are already on the manufacturer's. Drug, strength, date, time drawn and the initials of the person who prepared the syringe. As stated in the elements of performance for. Food and drug administration (fda), manufacturers of. Joint Commission Syringe Labeling Requirements.
From syringepumppro.com
Parts Of A Syringe SyringePumpPro Joint Commission Syringe Labeling Requirements Drug, strength, date, time drawn and the initials of the person who prepared the syringe. The only requirements for labeling include the name, strength, amount, and expiration date that are already on the manufacturer's. Food and drug administration (fda), manufacturers of certain medical devices and products must include. Syringes require 5 labeling elements: As stated in the elements of performance. Joint Commission Syringe Labeling Requirements.
From vigilantsoftware.wordpress.com
Syringe labels Site Title Joint Commission Syringe Labeling Requirements Syringes require 5 labeling elements: Drug, strength, date, time drawn and the initials of the person who prepared the syringe. National patient safety goal (npsg) 03.04.01, to improve the safety of using medications, was first. Food and drug administration (fda), manufacturers of certain medical devices and products must include. All solutions on the sterile field, including medications, saline, and/or water,. Joint Commission Syringe Labeling Requirements.
From surveylabel.blogspot.com
43 syringe labeled parts Joint Commission Syringe Labeling Requirements Food and drug administration (fda), manufacturers of certain medical devices and products must include. Home joint commission accreditation ambulatory national patient safety goals (npsg) standards. As stated in the elements of performance for. Requirements for labeling medications in the perioperative setting (joint commission, 2020). All solutions on the sterile field, including medications, saline, and/or water, should be labeled with name,. Joint Commission Syringe Labeling Requirements.
From guardianmed.net
Labeling Syringes while Reducing RSIs Guardian Medical Systems Joint Commission Syringe Labeling Requirements Requirements for labeling medications in the perioperative setting (joint commission, 2020). Food and drug administration (fda), manufacturers of certain medical devices and products must include. Syringes require 5 labeling elements: As stated in the elements of performance for. The only requirements for labeling include the name, strength, amount, and expiration date that are already on the manufacturer's. National patient safety. Joint Commission Syringe Labeling Requirements.
From www.medofficedirect.com
Syringe Buying Guide Joint Commission Syringe Labeling Requirements All solutions on the sterile field, including medications, saline, and/or water, should be labeled with name, strength, dosage, and. Home joint commission accreditation ambulatory national patient safety goals (npsg) standards. The only requirements for labeling include the name, strength, amount, and expiration date that are already on the manufacturer's. Syringes require 5 labeling elements: Drug, strength, date, time drawn and. Joint Commission Syringe Labeling Requirements.
From slidingmotion.com
Expert Guide of Syringe Parts Learn anatomy, functions & diagram Joint Commission Syringe Labeling Requirements Food and drug administration (fda), manufacturers of certain medical devices and products must include. National patient safety goal (npsg) 03.04.01, to improve the safety of using medications, was first. All solutions on the sterile field, including medications, saline, and/or water, should be labeled with name, strength, dosage, and. The only requirements for labeling include the name, strength, amount, and expiration. Joint Commission Syringe Labeling Requirements.
From www.youtube.com
" Functional Label and Labeling" Syringe Shrink Tack Label YouTube Joint Commission Syringe Labeling Requirements National patient safety goal (npsg) 03.04.01, to improve the safety of using medications, was first. Food and drug administration (fda), manufacturers of certain medical devices and products must include. The only requirements for labeling include the name, strength, amount, and expiration date that are already on the manufacturer's. All solutions on the sterile field, including medications, saline, and/or water, should. Joint Commission Syringe Labeling Requirements.
From healthycybersolutions.com
Syringe Labeling Healthy Cyber Solutions Joint Commission Syringe Labeling Requirements The only requirements for labeling include the name, strength, amount, and expiration date that are already on the manufacturer's. Drug, strength, date, time drawn and the initials of the person who prepared the syringe. Syringes require 5 labeling elements: Food and drug administration (fda), manufacturers of certain medical devices and products must include. Requirements for labeling medications in the perioperative. Joint Commission Syringe Labeling Requirements.
From gioydipcr.blob.core.windows.net
Joint Commission Labeling Syringes at Alice Cobb blog Joint Commission Syringe Labeling Requirements Syringes require 5 labeling elements: Drug, strength, date, time drawn and the initials of the person who prepared the syringe. As stated in the elements of performance for. All solutions on the sterile field, including medications, saline, and/or water, should be labeled with name, strength, dosage, and. National patient safety goal (npsg) 03.04.01, to improve the safety of using medications,. Joint Commission Syringe Labeling Requirements.
From quizlet.com
LA Lecture 4 Parts of a Syringe Labeling Diagram Quizlet Joint Commission Syringe Labeling Requirements All solutions on the sterile field, including medications, saline, and/or water, should be labeled with name, strength, dosage, and. Food and drug administration (fda), manufacturers of certain medical devices and products must include. Home joint commission accreditation ambulatory national patient safety goals (npsg) standards. Syringes require 5 labeling elements: The only requirements for labeling include the name, strength, amount, and. Joint Commission Syringe Labeling Requirements.
From darrin-cassell.blogspot.com
how to label a medication syringe darrincassell Joint Commission Syringe Labeling Requirements Food and drug administration (fda), manufacturers of certain medical devices and products must include. Syringes require 5 labeling elements: Home joint commission accreditation ambulatory national patient safety goals (npsg) standards. Drug, strength, date, time drawn and the initials of the person who prepared the syringe. The only requirements for labeling include the name, strength, amount, and expiration date that are. Joint Commission Syringe Labeling Requirements.
From quizlet.com
Syringe Parts Diagram Diagram Quizlet Joint Commission Syringe Labeling Requirements Drug, strength, date, time drawn and the initials of the person who prepared the syringe. Food and drug administration (fda), manufacturers of certain medical devices and products must include. Syringes require 5 labeling elements: As stated in the elements of performance for. The only requirements for labeling include the name, strength, amount, and expiration date that are already on the. Joint Commission Syringe Labeling Requirements.
From darrin-cassell.blogspot.com
how to label a medication syringe darrincassell Joint Commission Syringe Labeling Requirements Home joint commission accreditation ambulatory national patient safety goals (npsg) standards. All solutions on the sterile field, including medications, saline, and/or water, should be labeled with name, strength, dosage, and. Requirements for labeling medications in the perioperative setting (joint commission, 2020). Food and drug administration (fda), manufacturers of certain medical devices and products must include. The only requirements for labeling. Joint Commission Syringe Labeling Requirements.
From www.zoll.com
Joint Commission Resuscitation Standards ZOLL Medical Joint Commission Syringe Labeling Requirements Drug, strength, date, time drawn and the initials of the person who prepared the syringe. Requirements for labeling medications in the perioperative setting (joint commission, 2020). Home joint commission accreditation ambulatory national patient safety goals (npsg) standards. As stated in the elements of performance for. Food and drug administration (fda), manufacturers of certain medical devices and products must include. The. Joint Commission Syringe Labeling Requirements.
From medpak.com
How to Label a Medical Syringe Medical Packaging Inc. Joint Commission Syringe Labeling Requirements Drug, strength, date, time drawn and the initials of the person who prepared the syringe. Food and drug administration (fda), manufacturers of certain medical devices and products must include. As stated in the elements of performance for. All solutions on the sterile field, including medications, saline, and/or water, should be labeled with name, strength, dosage, and. National patient safety goal. Joint Commission Syringe Labeling Requirements.
From www.signelbiomedical.com
Comprehensive Guide for Syringe selection Understanding syringe Joint Commission Syringe Labeling Requirements Food and drug administration (fda), manufacturers of certain medical devices and products must include. National patient safety goal (npsg) 03.04.01, to improve the safety of using medications, was first. All solutions on the sterile field, including medications, saline, and/or water, should be labeled with name, strength, dosage, and. The only requirements for labeling include the name, strength, amount, and expiration. Joint Commission Syringe Labeling Requirements.
From www.youtube.com
Prefilled Syringe System YouTube Joint Commission Syringe Labeling Requirements The only requirements for labeling include the name, strength, amount, and expiration date that are already on the manufacturer's. National patient safety goal (npsg) 03.04.01, to improve the safety of using medications, was first. All solutions on the sterile field, including medications, saline, and/or water, should be labeled with name, strength, dosage, and. Drug, strength, date, time drawn and the. Joint Commission Syringe Labeling Requirements.
From www.slideserve.com
PPT The Joint Commission (TJC) and Practice Standards PowerPoint Presentation ID4465290 Joint Commission Syringe Labeling Requirements Syringes require 5 labeling elements: Food and drug administration (fda), manufacturers of certain medical devices and products must include. As stated in the elements of performance for. Drug, strength, date, time drawn and the initials of the person who prepared the syringe. Requirements for labeling medications in the perioperative setting (joint commission, 2020). All solutions on the sterile field, including. Joint Commission Syringe Labeling Requirements.
From surveylabel.blogspot.com
43 syringe labeled parts Joint Commission Syringe Labeling Requirements National patient safety goal (npsg) 03.04.01, to improve the safety of using medications, was first. Syringes require 5 labeling elements: Food and drug administration (fda), manufacturers of certain medical devices and products must include. As stated in the elements of performance for. All solutions on the sterile field, including medications, saline, and/or water, should be labeled with name, strength, dosage,. Joint Commission Syringe Labeling Requirements.
From bowesroegner-99.blogspot.com
how to label a medication syringe bowesroegner99 Joint Commission Syringe Labeling Requirements Requirements for labeling medications in the perioperative setting (joint commission, 2020). The only requirements for labeling include the name, strength, amount, and expiration date that are already on the manufacturer's. All solutions on the sterile field, including medications, saline, and/or water, should be labeled with name, strength, dosage, and. Syringes require 5 labeling elements: Home joint commission accreditation ambulatory national. Joint Commission Syringe Labeling Requirements.
From www.respiratorytherapyzone.com
Parts of a Syringe and Needle Components Explained (2023) Joint Commission Syringe Labeling Requirements Requirements for labeling medications in the perioperative setting (joint commission, 2020). Home joint commission accreditation ambulatory national patient safety goals (npsg) standards. The only requirements for labeling include the name, strength, amount, and expiration date that are already on the manufacturer's. As stated in the elements of performance for. Drug, strength, date, time drawn and the initials of the person. Joint Commission Syringe Labeling Requirements.
From dailymed.nlm.nih.gov
EPINEPHRINE INJ. USP, 0.1 mg/mL 1mg per 10mL LUERJET™ SYR Joint Commission Syringe Labeling Requirements Requirements for labeling medications in the perioperative setting (joint commission, 2020). Food and drug administration (fda), manufacturers of certain medical devices and products must include. Drug, strength, date, time drawn and the initials of the person who prepared the syringe. National patient safety goal (npsg) 03.04.01, to improve the safety of using medications, was first. All solutions on the sterile. Joint Commission Syringe Labeling Requirements.
From mungfali.com
How To Read Syringe Markings Joint Commission Syringe Labeling Requirements As stated in the elements of performance for. Drug, strength, date, time drawn and the initials of the person who prepared the syringe. Syringes require 5 labeling elements: The only requirements for labeling include the name, strength, amount, and expiration date that are already on the manufacturer's. All solutions on the sterile field, including medications, saline, and/or water, should be. Joint Commission Syringe Labeling Requirements.
From bundle.report
2023 Joint Commission ORYX Requirements Bundle Report Joint Commission Syringe Labeling Requirements All solutions on the sterile field, including medications, saline, and/or water, should be labeled with name, strength, dosage, and. Food and drug administration (fda), manufacturers of certain medical devices and products must include. The only requirements for labeling include the name, strength, amount, and expiration date that are already on the manufacturer's. As stated in the elements of performance for.. Joint Commission Syringe Labeling Requirements.
From www.youtube.com
How to label a syringe Dr.S.Parthasarathy MD., DNB, PhD YouTube Joint Commission Syringe Labeling Requirements As stated in the elements of performance for. National patient safety goal (npsg) 03.04.01, to improve the safety of using medications, was first. All solutions on the sterile field, including medications, saline, and/or water, should be labeled with name, strength, dosage, and. Drug, strength, date, time drawn and the initials of the person who prepared the syringe. Requirements for labeling. Joint Commission Syringe Labeling Requirements.
From www.labeltemplate.net
5 The Complete Guide to Syringe Labels & How They are Keeping You Protected label template Joint Commission Syringe Labeling Requirements All solutions on the sterile field, including medications, saline, and/or water, should be labeled with name, strength, dosage, and. Requirements for labeling medications in the perioperative setting (joint commission, 2020). As stated in the elements of performance for. Drug, strength, date, time drawn and the initials of the person who prepared the syringe. Food and drug administration (fda), manufacturers of. Joint Commission Syringe Labeling Requirements.
From www.ccianesthesia.com
NPSG Requirements for Labeling Syringes CCI Institute for Excellence Joint Commission Syringe Labeling Requirements All solutions on the sterile field, including medications, saline, and/or water, should be labeled with name, strength, dosage, and. The only requirements for labeling include the name, strength, amount, and expiration date that are already on the manufacturer's. Home joint commission accreditation ambulatory national patient safety goals (npsg) standards. As stated in the elements of performance for. National patient safety. Joint Commission Syringe Labeling Requirements.
From oralsyringes.uk
How to use a 5ml Oral Syringe Precision Oral Syringes Joint Commission Syringe Labeling Requirements The only requirements for labeling include the name, strength, amount, and expiration date that are already on the manufacturer's. All solutions on the sterile field, including medications, saline, and/or water, should be labeled with name, strength, dosage, and. Drug, strength, date, time drawn and the initials of the person who prepared the syringe. Food and drug administration (fda), manufacturers of. Joint Commission Syringe Labeling Requirements.
From www.apfireprotection.com
The Joint Commission, Fire Protection & YOU Everything You Need to Know about Fire Protection Joint Commission Syringe Labeling Requirements National patient safety goal (npsg) 03.04.01, to improve the safety of using medications, was first. The only requirements for labeling include the name, strength, amount, and expiration date that are already on the manufacturer's. As stated in the elements of performance for. Food and drug administration (fda), manufacturers of certain medical devices and products must include. Requirements for labeling medications. Joint Commission Syringe Labeling Requirements.
From www.scribd.com
Labeling of Syringes by Stephen M. Dorman, M.D PDF Joint Commission Medicine Joint Commission Syringe Labeling Requirements National patient safety goal (npsg) 03.04.01, to improve the safety of using medications, was first. Syringes require 5 labeling elements: Requirements for labeling medications in the perioperative setting (joint commission, 2020). As stated in the elements of performance for. The only requirements for labeling include the name, strength, amount, and expiration date that are already on the manufacturer's. Home joint. Joint Commission Syringe Labeling Requirements.