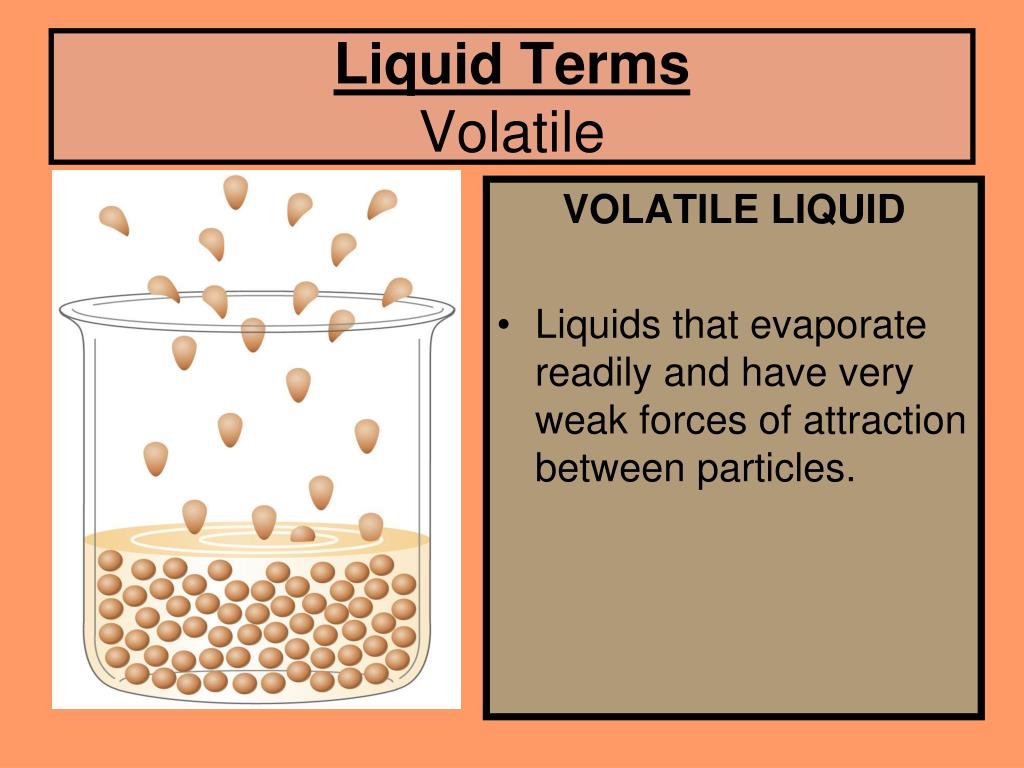
Surface Area For Liquid . The stronger the intermolecular forces, the greater the surface tension. The surface area is the amount of that liquid that is. Because of the unbalanced molecular attractions on the surface molecules, liquids contract to form a shape that minimizes the number of. Surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid. The stronger the intermolecular interactions, the. Surface tension is the energy required to increase the surface area of a liquid by a given amount. The surface tension is related to the force required to increase the surface area, and can explain why objects like a paper clip can float if they are on the surface, but sink if they are placed below the surface. The surface of a liquid is the interface between the liquid and (usually) the air surrounding it. Surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid surface by a given amount. Because of the unbalanced molecular attractions on the surface molecules, liquids contract to form a shape that minimizes the number of.
from www.slideserve.com
Surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid. The stronger the intermolecular interactions, the. The surface of a liquid is the interface between the liquid and (usually) the air surrounding it. Surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid surface by a given amount. The surface area is the amount of that liquid that is. Because of the unbalanced molecular attractions on the surface molecules, liquids contract to form a shape that minimizes the number of. Because of the unbalanced molecular attractions on the surface molecules, liquids contract to form a shape that minimizes the number of. Surface tension is the energy required to increase the surface area of a liquid by a given amount. The surface tension is related to the force required to increase the surface area, and can explain why objects like a paper clip can float if they are on the surface, but sink if they are placed below the surface. The stronger the intermolecular forces, the greater the surface tension.
PPT Chapter 10 Liquids and Solids PowerPoint Presentation, free
Surface Area For Liquid Because of the unbalanced molecular attractions on the surface molecules, liquids contract to form a shape that minimizes the number of. The stronger the intermolecular interactions, the. The surface area is the amount of that liquid that is. Surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid surface by a given amount. Because of the unbalanced molecular attractions on the surface molecules, liquids contract to form a shape that minimizes the number of. Surface tension is the energy required to increase the surface area of a liquid by a given amount. The surface of a liquid is the interface between the liquid and (usually) the air surrounding it. The stronger the intermolecular forces, the greater the surface tension. The surface tension is related to the force required to increase the surface area, and can explain why objects like a paper clip can float if they are on the surface, but sink if they are placed below the surface. Surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid. Because of the unbalanced molecular attractions on the surface molecules, liquids contract to form a shape that minimizes the number of.
From studylib.net
Chapter 11 Liquids and Solids A. Intermolecular Forces Surface Area For Liquid Surface tension is the energy required to increase the surface area of a liquid by a given amount. The surface of a liquid is the interface between the liquid and (usually) the air surrounding it. The surface tension is related to the force required to increase the surface area, and can explain why objects like a paper clip can float. Surface Area For Liquid.
From www.slideserve.com
PPT LIQUID SURFACES PowerPoint Presentation, free download ID4456243 Surface Area For Liquid The surface of a liquid is the interface between the liquid and (usually) the air surrounding it. Because of the unbalanced molecular attractions on the surface molecules, liquids contract to form a shape that minimizes the number of. The surface tension is related to the force required to increase the surface area, and can explain why objects like a paper. Surface Area For Liquid.
From ar.inspiredpencil.com
Surface Area Formula Sheet Surface Area For Liquid Surface tension is the energy required to increase the surface area of a liquid by a given amount. The surface tension is related to the force required to increase the surface area, and can explain why objects like a paper clip can float if they are on the surface, but sink if they are placed below the surface. The stronger. Surface Area For Liquid.
From physicsexperiments.eu
Dependence of Evaporation Rate of Liquid on Liquid Surface Area Surface Area For Liquid The stronger the intermolecular interactions, the. Because of the unbalanced molecular attractions on the surface molecules, liquids contract to form a shape that minimizes the number of. The surface tension is related to the force required to increase the surface area, and can explain why objects like a paper clip can float if they are on the surface, but sink. Surface Area For Liquid.
From www.slideserve.com
PPT Discussion Lecture(5) Chapter(3) Hydrostatic Forces on Surfaces Surface Area For Liquid Because of the unbalanced molecular attractions on the surface molecules, liquids contract to form a shape that minimizes the number of. Surface tension is the energy required to increase the surface area of a liquid by a given amount. The surface area is the amount of that liquid that is. The surface tension is related to the force required to. Surface Area For Liquid.
From www.slideserve.com
PPT Chapter 10 Liquids and Solids PowerPoint Presentation, free Surface Area For Liquid Surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid surface by a given amount. Surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid. The. Surface Area For Liquid.
From www.researchgate.net
Schematic of a liquid droplet on solid surface. (a) Young's wetting Surface Area For Liquid The stronger the intermolecular interactions, the. Because of the unbalanced molecular attractions on the surface molecules, liquids contract to form a shape that minimizes the number of. Surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid. The surface tension is related to. Surface Area For Liquid.
From teraznews.com
The Cone and the Cylinder Below Have Equal Surface Area Surface Area For Liquid The surface area is the amount of that liquid that is. Surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid. Surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase. Surface Area For Liquid.
From www.cuemath.com
Volume of Rectangular Tank Definition, Formula and Examples, Surface Area For Liquid The surface of a liquid is the interface between the liquid and (usually) the air surrounding it. Because of the unbalanced molecular attractions on the surface molecules, liquids contract to form a shape that minimizes the number of. The stronger the intermolecular interactions, the. Surface tension is the energy required to increase the surface area of a liquid by a. Surface Area For Liquid.
From fullwallpaper.neocities.org
Area Volume Surface Area For Liquid The surface tension is related to the force required to increase the surface area, and can explain why objects like a paper clip can float if they are on the surface, but sink if they are placed below the surface. The surface area is the amount of that liquid that is. The surface of a liquid is the interface between. Surface Area For Liquid.
From www.slideserve.com
PPT Chapter 13 Fluids PowerPoint Presentation ID350101 Surface Area For Liquid Surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid. The stronger the intermolecular interactions, the. The surface tension is related to the force required to increase the surface area, and can explain why objects like a paper clip can float if they. Surface Area For Liquid.
From www.slideserve.com
PPT LIQUID SURFACES PowerPoint Presentation, free download ID4456243 Surface Area For Liquid The surface of a liquid is the interface between the liquid and (usually) the air surrounding it. Because of the unbalanced molecular attractions on the surface molecules, liquids contract to form a shape that minimizes the number of. The stronger the intermolecular interactions, the. Surface tension is defined as the energy required to increase the surface area of a liquid,. Surface Area For Liquid.
From variasclasses.blogspot.com
Ncert Solutions For Class 9 Maths Chapter 13 Exercise 136 Várias Classes Surface Area For Liquid Surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid. Because of the unbalanced molecular attractions on the surface molecules, liquids contract to form a shape that minimizes the number of. The surface of a liquid is the interface between the liquid and. Surface Area For Liquid.
From sciencenotes.org
Liquid Definition Examples of Liquids Surface Area For Liquid The stronger the intermolecular interactions, the. Surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid surface by a given amount. The stronger the intermolecular forces, the greater the surface tension. Because of the unbalanced molecular attractions on the surface molecules, liquids contract. Surface Area For Liquid.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs Surface Area For Liquid Because of the unbalanced molecular attractions on the surface molecules, liquids contract to form a shape that minimizes the number of. The stronger the intermolecular forces, the greater the surface tension. The stronger the intermolecular interactions, the. Surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the. Surface Area For Liquid.
From www.cuemath.com
Surface Area Formulas Derivation, Examples Surface Area For Liquid The stronger the intermolecular forces, the greater the surface tension. Because of the unbalanced molecular attractions on the surface molecules, liquids contract to form a shape that minimizes the number of. The stronger the intermolecular interactions, the. Because of the unbalanced molecular attractions on the surface molecules, liquids contract to form a shape that minimizes the number of. Surface tension. Surface Area For Liquid.
From www.thoughtco.com
Liquid Definition and Examples (Chemistry) Surface Area For Liquid The surface tension is related to the force required to increase the surface area, and can explain why objects like a paper clip can float if they are on the surface, but sink if they are placed below the surface. Because of the unbalanced molecular attractions on the surface molecules, liquids contract to form a shape that minimizes the number. Surface Area For Liquid.
From www.slideserve.com
PPT Intermolecular Forces and Liquids and Solids PowerPoint Surface Area For Liquid The stronger the intermolecular interactions, the. The surface tension is related to the force required to increase the surface area, and can explain why objects like a paper clip can float if they are on the surface, but sink if they are placed below the surface. The surface area is the amount of that liquid that is. Because of the. Surface Area For Liquid.
From fluidmechanics-dbatu.blogspot.com
Inclined Plane Surface Submerged in Liquid Surface Area For Liquid The stronger the intermolecular forces, the greater the surface tension. Because of the unbalanced molecular attractions on the surface molecules, liquids contract to form a shape that minimizes the number of. Surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid surface by. Surface Area For Liquid.
From www.geeksforgeeks.org
Surface Area Formulas for Different Geometrical Figures Surface Area For Liquid The surface area is the amount of that liquid that is. The stronger the intermolecular forces, the greater the surface tension. Because of the unbalanced molecular attractions on the surface molecules, liquids contract to form a shape that minimizes the number of. The surface of a liquid is the interface between the liquid and (usually) the air surrounding it. The. Surface Area For Liquid.
From www.slideserve.com
PPT Properties of Liquids PowerPoint Presentation, free download ID Surface Area For Liquid Because of the unbalanced molecular attractions on the surface molecules, liquids contract to form a shape that minimizes the number of. Surface tension is the energy required to increase the surface area of a liquid by a given amount. The stronger the intermolecular interactions, the. The surface tension is related to the force required to increase the surface area, and. Surface Area For Liquid.
From cehivlda.blob.core.windows.net
How To Find The Surface Area Of A Rectangle at Luther Morton blog Surface Area For Liquid Surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid. The stronger the intermolecular interactions, the. Because of the unbalanced molecular attractions on the surface molecules, liquids contract to form a shape that minimizes the number of. The surface area is the amount. Surface Area For Liquid.
From www.slideserve.com
PPT LIQUID SURFACES PowerPoint Presentation, free download ID4456243 Surface Area For Liquid Surface tension is the energy required to increase the surface area of a liquid by a given amount. Surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid surface by a given amount. The stronger the intermolecular forces, the greater the surface tension.. Surface Area For Liquid.
From www.youtube.com
Pressure difference across liquid surfaces YouTube Surface Area For Liquid The surface area is the amount of that liquid that is. Surface tension is the energy required to increase the surface area of a liquid by a given amount. The stronger the intermolecular forces, the greater the surface tension. Because of the unbalanced molecular attractions on the surface molecules, liquids contract to form a shape that minimizes the number of.. Surface Area For Liquid.
From chem.libretexts.org
12.2 Some Properties of Liquids Chemistry LibreTexts Surface Area For Liquid The surface tension is related to the force required to increase the surface area, and can explain why objects like a paper clip can float if they are on the surface, but sink if they are placed below the surface. The stronger the intermolecular interactions, the. Surface tension is defined as the energy required to increase the surface area of. Surface Area For Liquid.
From learningschooltrkesp5v.z22.web.core.windows.net
Water Evaporation Rate By Temperature Surface Area For Liquid The surface of a liquid is the interface between the liquid and (usually) the air surrounding it. Because of the unbalanced molecular attractions on the surface molecules, liquids contract to form a shape that minimizes the number of. The surface tension is related to the force required to increase the surface area, and can explain why objects like a paper. Surface Area For Liquid.
From onlineengineeringnotes.com
Hydrostatic Forces on different submerged surface in liquid Surface Area For Liquid Because of the unbalanced molecular attractions on the surface molecules, liquids contract to form a shape that minimizes the number of. Surface tension is the energy required to increase the surface area of a liquid by a given amount. The surface area is the amount of that liquid that is. Because of the unbalanced molecular attractions on the surface molecules,. Surface Area For Liquid.
From www.flexiprep.com
NCERT Class 9 Solutions Surface Areas and Volumes (Chapter 13 Surface Area For Liquid The surface area is the amount of that liquid that is. Because of the unbalanced molecular attractions on the surface molecules, liquids contract to form a shape that minimizes the number of. Surface tension is the energy required to increase the surface area of a liquid by a given amount. Surface tension is defined as the energy required to increase. Surface Area For Liquid.
From www.youtube.com
Analysis of Hydrostatic Forces on Plane Surfaces YouTube Surface Area For Liquid The surface of a liquid is the interface between the liquid and (usually) the air surrounding it. The surface area is the amount of that liquid that is. The surface tension is related to the force required to increase the surface area, and can explain why objects like a paper clip can float if they are on the surface, but. Surface Area For Liquid.
From www.slideserve.com
PPT LIQUID SURFACES PowerPoint Presentation, free download ID4456243 Surface Area For Liquid The surface area is the amount of that liquid that is. The stronger the intermolecular forces, the greater the surface tension. Because of the unbalanced molecular attractions on the surface molecules, liquids contract to form a shape that minimizes the number of. The surface of a liquid is the interface between the liquid and (usually) the air surrounding it. Surface. Surface Area For Liquid.
From present5.com
Surface Chemistry Toolkit Making sense of the role Surface Area For Liquid The stronger the intermolecular interactions, the. The surface tension is related to the force required to increase the surface area, and can explain why objects like a paper clip can float if they are on the surface, but sink if they are placed below the surface. Surface tension is defined as the energy required to increase the surface area of. Surface Area For Liquid.
From pressbooks.bccampus.ca
11.4 Variation of Pressure with Depth in a Fluid College Physics Surface Area For Liquid Surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid. The stronger the intermolecular forces, the greater the surface tension. Surface tension is the energy required to increase the surface area of a liquid by a given amount. The surface area is the. Surface Area For Liquid.
From www.youtube.com
On the surface of liquids YouTube Surface Area For Liquid The stronger the intermolecular forces, the greater the surface tension. Because of the unbalanced molecular attractions on the surface molecules, liquids contract to form a shape that minimizes the number of. The surface area is the amount of that liquid that is. Surface tension is the energy required to increase the surface area of a liquid by a given amount.. Surface Area For Liquid.
From www.slideserve.com
PPT Discussion Lecture(4) Chapter(3) Hydrostatic Forces on Surfaces Surface Area For Liquid Because of the unbalanced molecular attractions on the surface molecules, liquids contract to form a shape that minimizes the number of. The surface of a liquid is the interface between the liquid and (usually) the air surrounding it. The stronger the intermolecular forces, the greater the surface tension. Surface tension is defined as the energy required to increase the surface. Surface Area For Liquid.
From www.slideserve.com
PPT The pressure of a liquid at rest depends only on gravity and the Surface Area For Liquid Because of the unbalanced molecular attractions on the surface molecules, liquids contract to form a shape that minimizes the number of. Surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid. Surface tension is the energy required to increase the surface area of. Surface Area For Liquid.