What Do You Mean By Acid Base Titration . The reagent (titrant) is the solution with a known molarity that will react with the analyte. The analyte (titrand) is the solution with an unknown molarity. The concentration of a solution can be determined by knowing the acid and base dissociation constant.
from general.chemistrysteps.com
The reagent (titrant) is the solution with a known molarity that will react with the analyte. The analyte (titrand) is the solution with an unknown molarity. The concentration of a solution can be determined by knowing the acid and base dissociation constant.
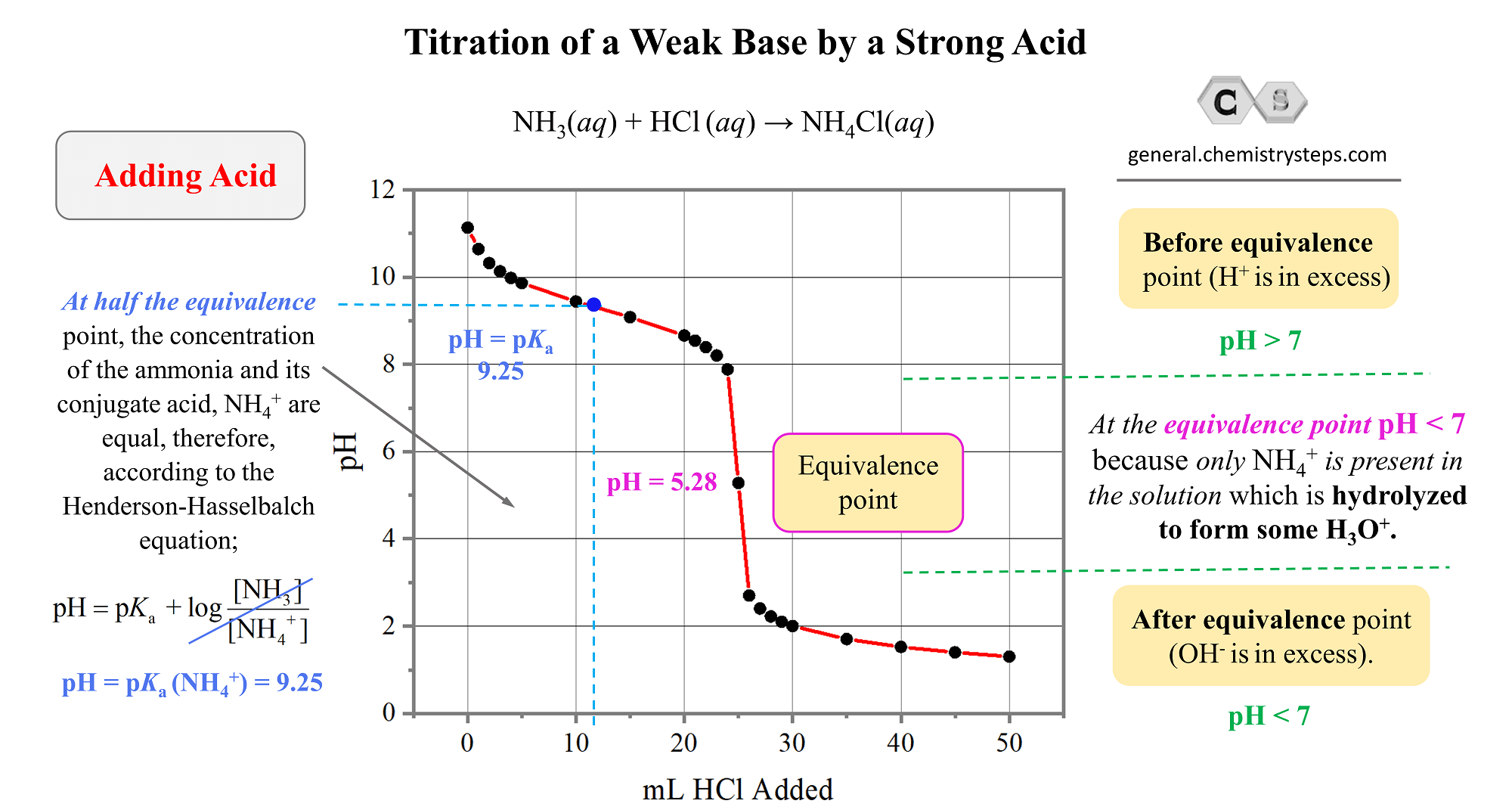
Titration of a Weak Base by a Strong Acid Chemistry Steps
What Do You Mean By Acid Base Titration The analyte (titrand) is the solution with an unknown molarity. The concentration of a solution can be determined by knowing the acid and base dissociation constant. The analyte (titrand) is the solution with an unknown molarity. The reagent (titrant) is the solution with a known molarity that will react with the analyte.
From printablehaferbrotwp.z21.web.core.windows.net
How To Do Titrations In Chemistry What Do You Mean By Acid Base Titration The analyte (titrand) is the solution with an unknown molarity. The concentration of a solution can be determined by knowing the acid and base dissociation constant. The reagent (titrant) is the solution with a known molarity that will react with the analyte. What Do You Mean By Acid Base Titration.
From www.slideserve.com
PPT Acid Base Titrations PowerPoint Presentation, free download ID What Do You Mean By Acid Base Titration The concentration of a solution can be determined by knowing the acid and base dissociation constant. The reagent (titrant) is the solution with a known molarity that will react with the analyte. The analyte (titrand) is the solution with an unknown molarity. What Do You Mean By Acid Base Titration.
From scienceinfo.com
Acidbase Titration 4 Types, Theory, Working Principle What Do You Mean By Acid Base Titration The analyte (titrand) is the solution with an unknown molarity. The reagent (titrant) is the solution with a known molarity that will react with the analyte. The concentration of a solution can be determined by knowing the acid and base dissociation constant. What Do You Mean By Acid Base Titration.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic What Do You Mean By Acid Base Titration The analyte (titrand) is the solution with an unknown molarity. The concentration of a solution can be determined by knowing the acid and base dissociation constant. The reagent (titrant) is the solution with a known molarity that will react with the analyte. What Do You Mean By Acid Base Titration.
From theedge.com.hk
Chemistry How To Titration The Edge What Do You Mean By Acid Base Titration The concentration of a solution can be determined by knowing the acid and base dissociation constant. The analyte (titrand) is the solution with an unknown molarity. The reagent (titrant) is the solution with a known molarity that will react with the analyte. What Do You Mean By Acid Base Titration.
From joixjswcw.blob.core.windows.net
Titration Of Acid Base Experiment at Joanne Rivera blog What Do You Mean By Acid Base Titration The reagent (titrant) is the solution with a known molarity that will react with the analyte. The analyte (titrand) is the solution with an unknown molarity. The concentration of a solution can be determined by knowing the acid and base dissociation constant. What Do You Mean By Acid Base Titration.
From www.slideserve.com
PPT AcidBase Titrations PowerPoint Presentation, free download ID What Do You Mean By Acid Base Titration The concentration of a solution can be determined by knowing the acid and base dissociation constant. The analyte (titrand) is the solution with an unknown molarity. The reagent (titrant) is the solution with a known molarity that will react with the analyte. What Do You Mean By Acid Base Titration.
From mungfali.com
Acid Base Titration Formula What Do You Mean By Acid Base Titration The analyte (titrand) is the solution with an unknown molarity. The concentration of a solution can be determined by knowing the acid and base dissociation constant. The reagent (titrant) is the solution with a known molarity that will react with the analyte. What Do You Mean By Acid Base Titration.
From saylordotorg.github.io
AcidBase Titrations What Do You Mean By Acid Base Titration The concentration of a solution can be determined by knowing the acid and base dissociation constant. The reagent (titrant) is the solution with a known molarity that will react with the analyte. The analyte (titrand) is the solution with an unknown molarity. What Do You Mean By Acid Base Titration.
From www.slideserve.com
PPT ACID BASE REACTIONS PowerPoint Presentation, free download ID What Do You Mean By Acid Base Titration The analyte (titrand) is the solution with an unknown molarity. The reagent (titrant) is the solution with a known molarity that will react with the analyte. The concentration of a solution can be determined by knowing the acid and base dissociation constant. What Do You Mean By Acid Base Titration.
From www.slideserve.com
PPT Chapter 14 Acids and Bases PowerPoint Presentation, free download What Do You Mean By Acid Base Titration The analyte (titrand) is the solution with an unknown molarity. The reagent (titrant) is the solution with a known molarity that will react with the analyte. The concentration of a solution can be determined by knowing the acid and base dissociation constant. What Do You Mean By Acid Base Titration.
From www.sciencephoto.com
Acidbase titration Stock Image C015/0435 Science Photo Library What Do You Mean By Acid Base Titration The concentration of a solution can be determined by knowing the acid and base dissociation constant. The reagent (titrant) is the solution with a known molarity that will react with the analyte. The analyte (titrand) is the solution with an unknown molarity. What Do You Mean By Acid Base Titration.
From www.studypool.com
SOLUTION Chemistry acid base titration Studypool What Do You Mean By Acid Base Titration The reagent (titrant) is the solution with a known molarity that will react with the analyte. The analyte (titrand) is the solution with an unknown molarity. The concentration of a solution can be determined by knowing the acid and base dissociation constant. What Do You Mean By Acid Base Titration.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators What Do You Mean By Acid Base Titration The analyte (titrand) is the solution with an unknown molarity. The reagent (titrant) is the solution with a known molarity that will react with the analyte. The concentration of a solution can be determined by knowing the acid and base dissociation constant. What Do You Mean By Acid Base Titration.
From www.studypool.com
SOLUTION Acid base titrations Studypool What Do You Mean By Acid Base Titration The analyte (titrand) is the solution with an unknown molarity. The reagent (titrant) is the solution with a known molarity that will react with the analyte. The concentration of a solution can be determined by knowing the acid and base dissociation constant. What Do You Mean By Acid Base Titration.
From www.youtube.com
Acids and Bases, Titrations, and Neutralization Reactions YouTube What Do You Mean By Acid Base Titration The reagent (titrant) is the solution with a known molarity that will react with the analyte. The concentration of a solution can be determined by knowing the acid and base dissociation constant. The analyte (titrand) is the solution with an unknown molarity. What Do You Mean By Acid Base Titration.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps What Do You Mean By Acid Base Titration The concentration of a solution can be determined by knowing the acid and base dissociation constant. The reagent (titrant) is the solution with a known molarity that will react with the analyte. The analyte (titrand) is the solution with an unknown molarity. What Do You Mean By Acid Base Titration.
From www.youtube.com
Acid Base Titration Curves pH Calculations YouTube What Do You Mean By Acid Base Titration The analyte (titrand) is the solution with an unknown molarity. The reagent (titrant) is the solution with a known molarity that will react with the analyte. The concentration of a solution can be determined by knowing the acid and base dissociation constant. What Do You Mean By Acid Base Titration.
From www.youtube.com
Introduction to ACIDBASE Titration Theory & Procedure YouTube What Do You Mean By Acid Base Titration The reagent (titrant) is the solution with a known molarity that will react with the analyte. The analyte (titrand) is the solution with an unknown molarity. The concentration of a solution can be determined by knowing the acid and base dissociation constant. What Do You Mean By Acid Base Titration.
From www.biorender.com
AcidBase Titration BioRender Science Templates What Do You Mean By Acid Base Titration The reagent (titrant) is the solution with a known molarity that will react with the analyte. The analyte (titrand) is the solution with an unknown molarity. The concentration of a solution can be determined by knowing the acid and base dissociation constant. What Do You Mean By Acid Base Titration.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations What Do You Mean By Acid Base Titration The concentration of a solution can be determined by knowing the acid and base dissociation constant. The reagent (titrant) is the solution with a known molarity that will react with the analyte. The analyte (titrand) is the solution with an unknown molarity. What Do You Mean By Acid Base Titration.
From www.youtube.com
Introduction to Acid Base Titrations YouTube What Do You Mean By Acid Base Titration The reagent (titrant) is the solution with a known molarity that will react with the analyte. The analyte (titrand) is the solution with an unknown molarity. The concentration of a solution can be determined by knowing the acid and base dissociation constant. What Do You Mean By Acid Base Titration.
From mungfali.com
Acid Base Titration Procedure What Do You Mean By Acid Base Titration The reagent (titrant) is the solution with a known molarity that will react with the analyte. The concentration of a solution can be determined by knowing the acid and base dissociation constant. The analyte (titrand) is the solution with an unknown molarity. What Do You Mean By Acid Base Titration.
From slideplayer.com
AcidBase Titrations AP Chemistry. ppt download What Do You Mean By Acid Base Titration The analyte (titrand) is the solution with an unknown molarity. The concentration of a solution can be determined by knowing the acid and base dissociation constant. The reagent (titrant) is the solution with a known molarity that will react with the analyte. What Do You Mean By Acid Base Titration.
From www.youtube.com
Acid Base Titration Experiment Acid base Titration Practical and What Do You Mean By Acid Base Titration The analyte (titrand) is the solution with an unknown molarity. The reagent (titrant) is the solution with a known molarity that will react with the analyte. The concentration of a solution can be determined by knowing the acid and base dissociation constant. What Do You Mean By Acid Base Titration.
From themasterchemistry.com
Acid Base TitrationWorking Principle, Process, Types And Indicators What Do You Mean By Acid Base Titration The reagent (titrant) is the solution with a known molarity that will react with the analyte. The analyte (titrand) is the solution with an unknown molarity. The concentration of a solution can be determined by knowing the acid and base dissociation constant. What Do You Mean By Acid Base Titration.
From www.slideserve.com
PPT AcidBase Titration PowerPoint Presentation, free download ID What Do You Mean By Acid Base Titration The reagent (titrant) is the solution with a known molarity that will react with the analyte. The concentration of a solution can be determined by knowing the acid and base dissociation constant. The analyte (titrand) is the solution with an unknown molarity. What Do You Mean By Acid Base Titration.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts What Do You Mean By Acid Base Titration The concentration of a solution can be determined by knowing the acid and base dissociation constant. The analyte (titrand) is the solution with an unknown molarity. The reagent (titrant) is the solution with a known molarity that will react with the analyte. What Do You Mean By Acid Base Titration.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple What Do You Mean By Acid Base Titration The reagent (titrant) is the solution with a known molarity that will react with the analyte. The analyte (titrand) is the solution with an unknown molarity. The concentration of a solution can be determined by knowing the acid and base dissociation constant. What Do You Mean By Acid Base Titration.
From www.slideserve.com
PPT AcidBase Titrations PowerPoint Presentation, free download ID What Do You Mean By Acid Base Titration The reagent (titrant) is the solution with a known molarity that will react with the analyte. The analyte (titrand) is the solution with an unknown molarity. The concentration of a solution can be determined by knowing the acid and base dissociation constant. What Do You Mean By Acid Base Titration.
From www.sliderbase.com
Titration What Do You Mean By Acid Base Titration The analyte (titrand) is the solution with an unknown molarity. The concentration of a solution can be determined by knowing the acid and base dissociation constant. The reagent (titrant) is the solution with a known molarity that will react with the analyte. What Do You Mean By Acid Base Titration.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps What Do You Mean By Acid Base Titration The analyte (titrand) is the solution with an unknown molarity. The reagent (titrant) is the solution with a known molarity that will react with the analyte. The concentration of a solution can be determined by knowing the acid and base dissociation constant. What Do You Mean By Acid Base Titration.
From mungfali.com
Acid Base Titration Calculation What Do You Mean By Acid Base Titration The reagent (titrant) is the solution with a known molarity that will react with the analyte. The concentration of a solution can be determined by knowing the acid and base dissociation constant. The analyte (titrand) is the solution with an unknown molarity. What Do You Mean By Acid Base Titration.
From www.youtube.com
AcidBase Titration Equivalence Point YouTube What Do You Mean By Acid Base Titration The reagent (titrant) is the solution with a known molarity that will react with the analyte. The concentration of a solution can be determined by knowing the acid and base dissociation constant. The analyte (titrand) is the solution with an unknown molarity. What Do You Mean By Acid Base Titration.
From stock.adobe.com
Acid base titration experiment and phases of color change during What Do You Mean By Acid Base Titration The reagent (titrant) is the solution with a known molarity that will react with the analyte. The analyte (titrand) is the solution with an unknown molarity. The concentration of a solution can be determined by knowing the acid and base dissociation constant. What Do You Mean By Acid Base Titration.