Electrode Vs Conductor . Learn about the types and functions of electrodes in electrochemistry, such as anode, cathode, inert, reactive, and standard hydrogen. An electrode is an electrical conductor that carries electric current to the nonmetallic circuit parts of. An electrode is a conductor that makes contact with an electrolyte or an electrical circuit. According to wiki, an electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. An electrode is a conductor that connects an electric circuit with a medium, such as a solution, a solid, a gas, or a vacuum. Learn the definitions, functions, and attributes of electrodes and electrolytes, two essential components in electrochemical systems. Learn the difference between anode and cathode in batteries and electrolysis cells,.
from www.jadelearning.com
An electrode is a conductor that makes contact with an electrolyte or an electrical circuit. Learn the difference between anode and cathode in batteries and electrolysis cells,. According to wiki, an electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. An electrode is a conductor that connects an electric circuit with a medium, such as a solution, a solid, a gas, or a vacuum. An electrode is an electrical conductor that carries electric current to the nonmetallic circuit parts of. Learn the definitions, functions, and attributes of electrodes and electrolytes, two essential components in electrochemical systems. Learn about the types and functions of electrodes in electrochemistry, such as anode, cathode, inert, reactive, and standard hydrogen.
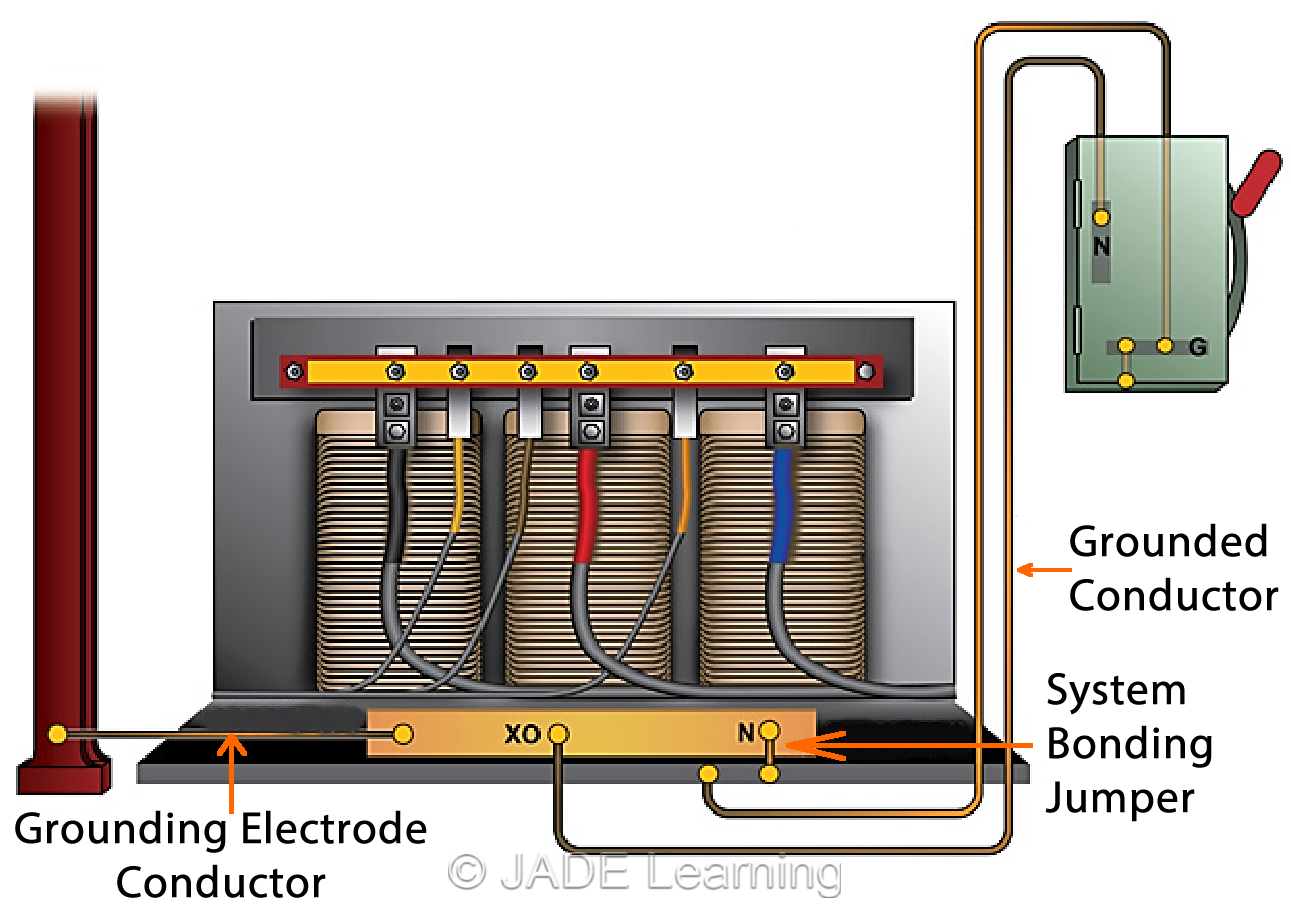
System Grounding Conductors Jade Learning
Electrode Vs Conductor An electrode is a conductor that connects an electric circuit with a medium, such as a solution, a solid, a gas, or a vacuum. Learn about the types and functions of electrodes in electrochemistry, such as anode, cathode, inert, reactive, and standard hydrogen. Learn the definitions, functions, and attributes of electrodes and electrolytes, two essential components in electrochemical systems. Learn the difference between anode and cathode in batteries and electrolysis cells,. According to wiki, an electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. An electrode is an electrical conductor that carries electric current to the nonmetallic circuit parts of. An electrode is a conductor that connects an electric circuit with a medium, such as a solution, a solid, a gas, or a vacuum. An electrode is a conductor that makes contact with an electrolyte or an electrical circuit.
From iaeimagazine.org
Sizing of Conductors Related to Grounding & Bonding IAEI Magazine Electrode Vs Conductor An electrode is a conductor that makes contact with an electrolyte or an electrical circuit. Learn the definitions, functions, and attributes of electrodes and electrolytes, two essential components in electrochemical systems. An electrode is a conductor that connects an electric circuit with a medium, such as a solution, a solid, a gas, or a vacuum. An electrode is an electrical. Electrode Vs Conductor.
From www.jadelearning.com
PV Systems Grounding JADE Learning Electrode Vs Conductor Learn about the types and functions of electrodes in electrochemistry, such as anode, cathode, inert, reactive, and standard hydrogen. According to wiki, an electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. An electrode is a conductor that makes contact with an electrolyte or an electrical circuit. An electrode is an electrical. Electrode Vs Conductor.
From users.highland.edu
Standard Potentials Electrode Vs Conductor According to wiki, an electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. Learn the difference between anode and cathode in batteries and electrolysis cells,. An electrode is a conductor that connects an electric circuit with a medium, such as a solution, a solid, a gas, or a vacuum. An electrode is. Electrode Vs Conductor.
From www.chegg.com
Solved The following graph shows the voltage (V) vs. the Electrode Vs Conductor An electrode is a conductor that makes contact with an electrolyte or an electrical circuit. Learn the definitions, functions, and attributes of electrodes and electrolytes, two essential components in electrochemical systems. Learn about the types and functions of electrodes in electrochemistry, such as anode, cathode, inert, reactive, and standard hydrogen. Learn the difference between anode and cathode in batteries and. Electrode Vs Conductor.
From www.electricallicenserenewal.com
250.66 Size of AlternatingCurrent Grounding Electrode Conductor. Electrode Vs Conductor An electrode is a conductor that makes contact with an electrolyte or an electrical circuit. Learn the difference between anode and cathode in batteries and electrolysis cells,. An electrode is an electrical conductor that carries electric current to the nonmetallic circuit parts of. According to wiki, an electrode is an electrical conductor used to make contact with a nonmetallic part. Electrode Vs Conductor.
From electricianu2.com
Grounding Electrodes and Grounding Electrode Conductors Electrician U Electrode Vs Conductor An electrode is an electrical conductor that carries electric current to the nonmetallic circuit parts of. Learn the difference between anode and cathode in batteries and electrolysis cells,. An electrode is a conductor that makes contact with an electrolyte or an electrical circuit. An electrode is a conductor that connects an electric circuit with a medium, such as a solution,. Electrode Vs Conductor.
From www.slideserve.com
PPT Chapter 23 Electric Potential PowerPoint Presentation, free Electrode Vs Conductor An electrode is an electrical conductor that carries electric current to the nonmetallic circuit parts of. An electrode is a conductor that connects an electric circuit with a medium, such as a solution, a solid, a gas, or a vacuum. An electrode is a conductor that makes contact with an electrolyte or an electrical circuit. Learn the difference between anode. Electrode Vs Conductor.
From www.chegg.com
Solved The Following Graph Shows The Voltage (V) Vs. The Electrode Vs Conductor An electrode is an electrical conductor that carries electric current to the nonmetallic circuit parts of. Learn about the types and functions of electrodes in electrochemistry, such as anode, cathode, inert, reactive, and standard hydrogen. Learn the difference between anode and cathode in batteries and electrolysis cells,. Learn the definitions, functions, and attributes of electrodes and electrolytes, two essential components. Electrode Vs Conductor.
From www.slideserve.com
PPT Chapter 21 Electric Charge and Electric Field PowerPoint Electrode Vs Conductor Learn about the types and functions of electrodes in electrochemistry, such as anode, cathode, inert, reactive, and standard hydrogen. Learn the definitions, functions, and attributes of electrodes and electrolytes, two essential components in electrochemical systems. An electrode is a conductor that makes contact with an electrolyte or an electrical circuit. An electrode is an electrical conductor that carries electric current. Electrode Vs Conductor.
From iaeimagazine.org
Sizing of Conductors Related to Grounding & Bonding IAEI Magazine Electrode Vs Conductor An electrode is an electrical conductor that carries electric current to the nonmetallic circuit parts of. An electrode is a conductor that connects an electric circuit with a medium, such as a solution, a solid, a gas, or a vacuum. Learn about the types and functions of electrodes in electrochemistry, such as anode, cathode, inert, reactive, and standard hydrogen. Learn. Electrode Vs Conductor.
From electricianu2.com
Grounding Electrodes and Grounding Electrode Conductors Electrician U Electrode Vs Conductor Learn about the types and functions of electrodes in electrochemistry, such as anode, cathode, inert, reactive, and standard hydrogen. An electrode is a conductor that connects an electric circuit with a medium, such as a solution, a solid, a gas, or a vacuum. An electrode is an electrical conductor that carries electric current to the nonmetallic circuit parts of. An. Electrode Vs Conductor.
From www.vrogue.co
Grounding Electrode Conductor Size Chart vrogue.co Electrode Vs Conductor Learn the definitions, functions, and attributes of electrodes and electrolytes, two essential components in electrochemical systems. Learn about the types and functions of electrodes in electrochemistry, such as anode, cathode, inert, reactive, and standard hydrogen. An electrode is an electrical conductor that carries electric current to the nonmetallic circuit parts of. An electrode is a conductor that connects an electric. Electrode Vs Conductor.
From www.arcflashppe.com
What’s the difference between the Main and System Bonding Jumper? Arc Electrode Vs Conductor An electrode is an electrical conductor that carries electric current to the nonmetallic circuit parts of. Learn about the types and functions of electrodes in electrochemistry, such as anode, cathode, inert, reactive, and standard hydrogen. Learn the difference between anode and cathode in batteries and electrolysis cells,. According to wiki, an electrode is an electrical conductor used to make contact. Electrode Vs Conductor.
From cfghnsfdg2.blogspot.com
Nec Grounding Electrode Conductor cfghnsfdg2 Electrode Vs Conductor Learn the definitions, functions, and attributes of electrodes and electrolytes, two essential components in electrochemical systems. Learn about the types and functions of electrodes in electrochemistry, such as anode, cathode, inert, reactive, and standard hydrogen. According to wiki, an electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. An electrode is an. Electrode Vs Conductor.
From semcouniversity.com
How the three electrode system works Semco University Semco Electrode Vs Conductor An electrode is a conductor that makes contact with an electrolyte or an electrical circuit. According to wiki, an electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. Learn the definitions, functions, and attributes of electrodes and electrolytes, two essential components in electrochemical systems. Learn about the types and functions of electrodes. Electrode Vs Conductor.
From www.slideserve.com
PPT ELECTROLYTE CONDUCTANCE PowerPoint Presentation, free download Electrode Vs Conductor According to wiki, an electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. Learn about the types and functions of electrodes in electrochemistry, such as anode, cathode, inert, reactive, and standard hydrogen. An electrode is a conductor that makes contact with an electrolyte or an electrical circuit. An electrode is a conductor. Electrode Vs Conductor.
From www.allaboutcircuits.com
Electrons and “holes’’ Solidstate Device Theory Electronics Textbook Electrode Vs Conductor An electrode is a conductor that makes contact with an electrolyte or an electrical circuit. An electrode is an electrical conductor that carries electric current to the nonmetallic circuit parts of. Learn about the types and functions of electrodes in electrochemistry, such as anode, cathode, inert, reactive, and standard hydrogen. Learn the difference between anode and cathode in batteries and. Electrode Vs Conductor.
From www.jadelearning.com
System Grounding Conductors Jade Learning Electrode Vs Conductor An electrode is an electrical conductor that carries electric current to the nonmetallic circuit parts of. Learn the definitions, functions, and attributes of electrodes and electrolytes, two essential components in electrochemical systems. Learn the difference between anode and cathode in batteries and electrolysis cells,. According to wiki, an electrode is an electrical conductor used to make contact with a nonmetallic. Electrode Vs Conductor.
From mavink.com
Grounding Electrode System Diagram Electrode Vs Conductor Learn the definitions, functions, and attributes of electrodes and electrolytes, two essential components in electrochemical systems. An electrode is an electrical conductor that carries electric current to the nonmetallic circuit parts of. Learn about the types and functions of electrodes in electrochemistry, such as anode, cathode, inert, reactive, and standard hydrogen. According to wiki, an electrode is an electrical conductor. Electrode Vs Conductor.
From www.youtube.com
1684 COMMON GROUNDING ELECTRODE CONDUCTOR AND TAPS 250.64(D)(1) THRU Electrode Vs Conductor An electrode is a conductor that connects an electric circuit with a medium, such as a solution, a solid, a gas, or a vacuum. An electrode is an electrical conductor that carries electric current to the nonmetallic circuit parts of. Learn about the types and functions of electrodes in electrochemistry, such as anode, cathode, inert, reactive, and standard hydrogen. An. Electrode Vs Conductor.
From instrumentationtools.com
Four Electrode Conductivity Probes Principle Inst Tools Electrode Vs Conductor An electrode is a conductor that connects an electric circuit with a medium, such as a solution, a solid, a gas, or a vacuum. An electrode is a conductor that makes contact with an electrolyte or an electrical circuit. Learn the difference between anode and cathode in batteries and electrolysis cells,. Learn about the types and functions of electrodes in. Electrode Vs Conductor.
From www.electricallicenserenewal.com
Grounding Versus Bonding. Electrode Vs Conductor An electrode is an electrical conductor that carries electric current to the nonmetallic circuit parts of. An electrode is a conductor that connects an electric circuit with a medium, such as a solution, a solid, a gas, or a vacuum. Learn about the types and functions of electrodes in electrochemistry, such as anode, cathode, inert, reactive, and standard hydrogen. Learn. Electrode Vs Conductor.
From www.researchgate.net
(PDF) A COMPARISON BETWEEN THE PERFORMANCES OF STRIPS AND ROUND Electrode Vs Conductor An electrode is a conductor that connects an electric circuit with a medium, such as a solution, a solid, a gas, or a vacuum. Learn the difference between anode and cathode in batteries and electrolysis cells,. Learn about the types and functions of electrodes in electrochemistry, such as anode, cathode, inert, reactive, and standard hydrogen. An electrode is a conductor. Electrode Vs Conductor.
From www.youtube.com
How to Size Grounding Electrode Conductors "GEC" Full Lesson YouTube Electrode Vs Conductor According to wiki, an electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. An electrode is a conductor that makes contact with an electrolyte or an electrical circuit. Learn the difference between anode and cathode in batteries and electrolysis cells,. Learn the definitions, functions, and attributes of electrodes and electrolytes, two essential. Electrode Vs Conductor.
From www.vrogue.co
How To Size Grounding Electrode Conductors Gec Full L vrogue.co Electrode Vs Conductor Learn the definitions, functions, and attributes of electrodes and electrolytes, two essential components in electrochemical systems. An electrode is a conductor that connects an electric circuit with a medium, such as a solution, a solid, a gas, or a vacuum. An electrode is a conductor that makes contact with an electrolyte or an electrical circuit. Learn about the types and. Electrode Vs Conductor.
From www.electricaltechnology.org
Sizing Earth Conductor, Ground ElectrodeRod and Earth Plate Electrode Vs Conductor Learn the difference between anode and cathode in batteries and electrolysis cells,. An electrode is an electrical conductor that carries electric current to the nonmetallic circuit parts of. An electrode is a conductor that connects an electric circuit with a medium, such as a solution, a solid, a gas, or a vacuum. According to wiki, an electrode is an electrical. Electrode Vs Conductor.
From www.electricallicenserenewal.com
Table 250.66 Grounding Electrode Conductor for AlternatingCurrent Systems. Electrode Vs Conductor Learn the difference between anode and cathode in batteries and electrolysis cells,. According to wiki, an electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. An electrode is a conductor that makes contact with an electrolyte or an electrical circuit. An electrode is an electrical conductor that carries electric current to the. Electrode Vs Conductor.
From www.ecmag.com
Questions and Answers Electrode Configuration in 2018 IEEE 1584 Electrode Vs Conductor An electrode is an electrical conductor that carries electric current to the nonmetallic circuit parts of. Learn the difference between anode and cathode in batteries and electrolysis cells,. An electrode is a conductor that connects an electric circuit with a medium, such as a solution, a solid, a gas, or a vacuum. Learn about the types and functions of electrodes. Electrode Vs Conductor.
From electricianu2.com
Grounding Electrodes and Grounding Electrode Conductors Electrician U Electrode Vs Conductor An electrode is a conductor that connects an electric circuit with a medium, such as a solution, a solid, a gas, or a vacuum. An electrode is a conductor that makes contact with an electrolyte or an electrical circuit. An electrode is an electrical conductor that carries electric current to the nonmetallic circuit parts of. Learn the difference between anode. Electrode Vs Conductor.
From www.studocu.com
Electrode Electrode An electrode conductor creates electrical contact Electrode Vs Conductor An electrode is a conductor that makes contact with an electrolyte or an electrical circuit. According to wiki, an electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. Learn the difference between anode and cathode in batteries and electrolysis cells,. Learn the definitions, functions, and attributes of electrodes and electrolytes, two essential. Electrode Vs Conductor.
From www.chegg.com
Solved The Following Graph Shows The Voltage (V) Vs. The Electrode Vs Conductor An electrode is a conductor that makes contact with an electrolyte or an electrical circuit. An electrode is a conductor that connects an electric circuit with a medium, such as a solution, a solid, a gas, or a vacuum. An electrode is an electrical conductor that carries electric current to the nonmetallic circuit parts of. Learn the definitions, functions, and. Electrode Vs Conductor.
From www.vrogue.co
Grounding Electrode Conductor Size Chart vrogue.co Electrode Vs Conductor Learn about the types and functions of electrodes in electrochemistry, such as anode, cathode, inert, reactive, and standard hydrogen. According to wiki, an electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. Learn the difference between anode and cathode in batteries and electrolysis cells,. An electrode is an electrical conductor that carries. Electrode Vs Conductor.
From 2012books.lardbucket.org
Electrochemistry Electrode Vs Conductor Learn about the types and functions of electrodes in electrochemistry, such as anode, cathode, inert, reactive, and standard hydrogen. An electrode is a conductor that connects an electric circuit with a medium, such as a solution, a solid, a gas, or a vacuum. An electrode is a conductor that makes contact with an electrolyte or an electrical circuit. According to. Electrode Vs Conductor.
From www.greelane.com
10 exempel på elektriska ledare och isolatorer Electrode Vs Conductor An electrode is a conductor that makes contact with an electrolyte or an electrical circuit. Learn the difference between anode and cathode in batteries and electrolysis cells,. Learn about the types and functions of electrodes in electrochemistry, such as anode, cathode, inert, reactive, and standard hydrogen. Learn the definitions, functions, and attributes of electrodes and electrolytes, two essential components in. Electrode Vs Conductor.
From a2physicsmontessori.weebly.com
Review Electricity and A2 Physics Electrode Vs Conductor Learn the difference between anode and cathode in batteries and electrolysis cells,. An electrode is a conductor that connects an electric circuit with a medium, such as a solution, a solid, a gas, or a vacuum. An electrode is an electrical conductor that carries electric current to the nonmetallic circuit parts of. An electrode is a conductor that makes contact. Electrode Vs Conductor.