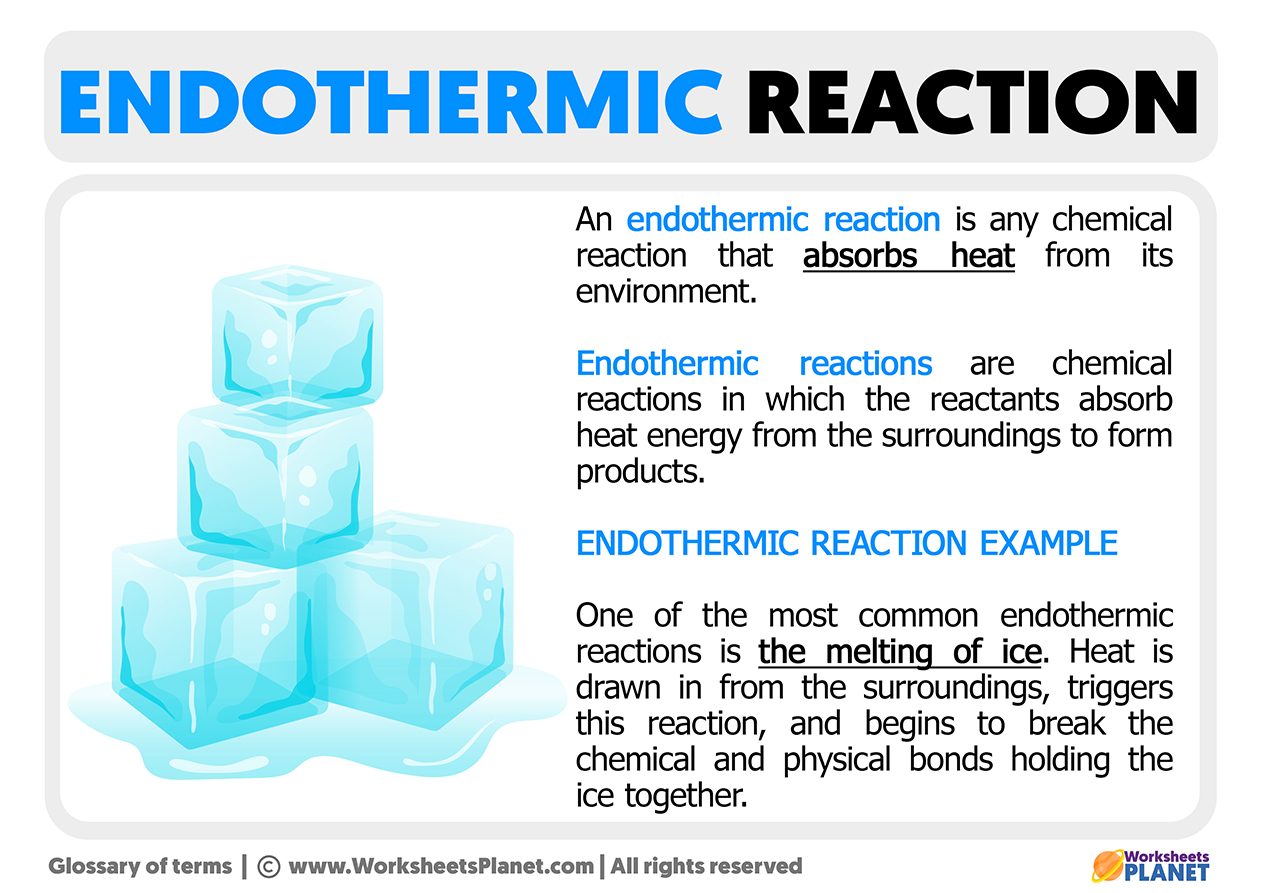
Endothermic Reaction Wikipedia . An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. The opposite is an endothermic reaction, which usually takes up heat and is driven by an entropy increase in the system. An endothermic reaction is a reaction between two elements or compounds that absorbs heat energy. Because heat is absorbed, endothermic. In an endothermic reaction, the substance. The opposite of an endothermic reaction is an. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. An endothermic reaction is a chemical reaction that takes in energy from the surroundings. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their surroundings.
from www.worksheetsplanet.com
An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. The opposite is an endothermic reaction, which usually takes up heat and is driven by an entropy increase in the system. The opposite of an endothermic reaction is an. An endothermic reaction is a chemical reaction that takes in energy from the surroundings. Because heat is absorbed, endothermic. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their surroundings. An endothermic reaction is a reaction between two elements or compounds that absorbs heat energy. In an endothermic reaction, the substance.
What is an Endothermic Reaction Definition & Example
Endothermic Reaction Wikipedia An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. In an endothermic reaction, the substance. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their surroundings. An endothermic reaction is a chemical reaction that takes in energy from the surroundings. The opposite of an endothermic reaction is an. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. Because heat is absorbed, endothermic. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. The opposite is an endothermic reaction, which usually takes up heat and is driven by an entropy increase in the system. An endothermic reaction is a reaction between two elements or compounds that absorbs heat energy.
From web.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Endothermic Endothermic Reaction Wikipedia For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. An endothermic reaction is a reaction between two elements or compounds that absorbs heat energy. An endothermic reaction is a chemical reaction that takes in energy from the surroundings. Because heat is absorbed, endothermic. An endothermic reaction is a chemical. Endothermic Reaction Wikipedia.
From slideplayer.com
Endothermic & Exothermic Reactions ppt download Endothermic Reaction Wikipedia The opposite is an endothermic reaction, which usually takes up heat and is driven by an entropy increase in the system. The opposite of an endothermic reaction is an. In an endothermic reaction, the substance. An endothermic reaction is a reaction between two elements or compounds that absorbs heat energy. For an endothermic chemical reaction to proceed, the reactants must. Endothermic Reaction Wikipedia.
From whatisdiffer.com
Difference Between Endothermic And Exothermic Reactions? Endothermic Reaction Wikipedia An endothermic reaction is a chemical reaction that takes in energy from the surroundings. Because heat is absorbed, endothermic. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their surroundings. The opposite is an endothermic reaction, which usually takes up heat and is driven by an entropy increase in. Endothermic Reaction Wikipedia.
From www.teachoo.com
Which of the reactions is an endothermic reaction? MCQ Science Endothermic Reaction Wikipedia An endothermic reaction is a reaction between two elements or compounds that absorbs heat energy. The opposite is an endothermic reaction, which usually takes up heat and is driven by an entropy increase in the system. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. In an endothermic reaction, the substance. Because heat is absorbed,. Endothermic Reaction Wikipedia.
From www.slideserve.com
PPT Unit 4 PowerPoint Presentation, free download ID7003399 Endothermic Reaction Wikipedia An endothermic reaction is a reaction between two elements or compounds that absorbs heat energy. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their surroundings. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. Because heat is. Endothermic Reaction Wikipedia.
From eduinput.com
Endothermic ReactionsCharacteristics, Identification, and Examples Endothermic Reaction Wikipedia An endothermic reaction is a chemical reaction that takes in energy from the surroundings. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. An endothermic reaction is a reaction between two elements or compounds that absorbs heat energy. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer. Endothermic Reaction Wikipedia.
From www.difference.wiki
Endothermic Reactions vs. Exothermic Reactions What’s the Difference? Endothermic Reaction Wikipedia Because heat is absorbed, endothermic. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. The opposite of an endothermic reaction is an. An endothermic reaction is a chemical reaction that takes in energy from the. Endothermic Reaction Wikipedia.
From www.difference.wiki
Exothermic Reaction vs. Endothermic Reaction What’s the Difference? Endothermic Reaction Wikipedia For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. The opposite of an endothermic reaction is an. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their surroundings. Because heat is absorbed, endothermic. An endothermic reaction is a. Endothermic Reaction Wikipedia.
From www.slideserve.com
PPT SCIENCE REVIEW PowerPoint Presentation, free download ID2375833 Endothermic Reaction Wikipedia The opposite of an endothermic reaction is an. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their surroundings. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to. Endothermic Reaction Wikipedia.
From www.slideserve.com
PPT Chemical Reactions PowerPoint Presentation, free download ID Endothermic Reaction Wikipedia An endothermic reaction is a chemical reaction that takes in energy from the surroundings. Because heat is absorbed, endothermic. The opposite is an endothermic reaction, which usually takes up heat and is driven by an entropy increase in the system. An endothermic reaction is a reaction between two elements or compounds that absorbs heat energy. For an endothermic chemical reaction. Endothermic Reaction Wikipedia.
From testbook.com
Endothermic Reaction Learn Definition, Reagents, Formula here Endothermic Reaction Wikipedia Because heat is absorbed, endothermic. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. An endothermic reaction is a reaction between two elements or compounds that absorbs heat energy. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. In an endothermic reaction, the substance.. Endothermic Reaction Wikipedia.
From www.slideserve.com
PPT Phase Changes PowerPoint Presentation, free download ID2202556 Endothermic Reaction Wikipedia Because heat is absorbed, endothermic. An endothermic reaction is a reaction between two elements or compounds that absorbs heat energy. The opposite of an endothermic reaction is an. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their surroundings. The opposite is an endothermic reaction, which usually takes up. Endothermic Reaction Wikipedia.
From www.slideserve.com
PPT Chapter 12 “Heat in Chemical Reactions” PowerPoint Presentation Endothermic Reaction Wikipedia An endothermic reaction is a reaction between two elements or compounds that absorbs heat energy. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their surroundings. The opposite of an endothermic reaction is an. Because heat is absorbed, endothermic. For an endothermic chemical reaction to proceed, the reactants must. Endothermic Reaction Wikipedia.
From www.slideserve.com
PPT Chemical Reactions PowerPoint Presentation, free download ID431456 Endothermic Reaction Wikipedia Because heat is absorbed, endothermic. In an endothermic reaction, the substance. An endothermic reaction is a reaction between two elements or compounds that absorbs heat energy. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. An endothermic reaction is a chemical reaction that takes in energy from the surroundings. Revise and understand what endothermic and. Endothermic Reaction Wikipedia.
From www.youtube.com
Endothermic Reactions YouTube Endothermic Reaction Wikipedia Because heat is absorbed, endothermic. The opposite is an endothermic reaction, which usually takes up heat and is driven by an entropy increase in the system. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products.. Endothermic Reaction Wikipedia.
From classnotes123.com
What does one mean by exothermic and endothermic reactions? Give Endothermic Reaction Wikipedia In an endothermic reaction, the substance. Because heat is absorbed, endothermic. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their surroundings. The opposite of an endothermic reaction is an. The opposite is an endothermic reaction, which usually takes up heat and is driven by an entropy increase in. Endothermic Reaction Wikipedia.
From www.slideserve.com
PPT Activity 8 Chemical Energy PowerPoint Presentation, free download Endothermic Reaction Wikipedia An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. In an endothermic reaction, the substance. The opposite of an endothermic reaction is an. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. An endothermic reaction is a chemical reaction that takes in energy from. Endothermic Reaction Wikipedia.
From slideplayer.com
Chemistry Notes Chapter 2 ppt download Endothermic Reaction Wikipedia In an endothermic reaction, the substance. An endothermic reaction is a chemical reaction that takes in energy from the surroundings. Because heat is absorbed, endothermic. The opposite is an endothermic reaction, which usually takes up heat and is driven by an entropy increase in the system. The opposite of an endothermic reaction is an. For an endothermic chemical reaction to. Endothermic Reaction Wikipedia.
From mungfali.com
Endothermic Diagram With Labels Endothermic Reaction Wikipedia The opposite of an endothermic reaction is an. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. Because heat is absorbed, endothermic. In an endothermic reaction, the substance. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. The opposite is an endothermic reaction, which. Endothermic Reaction Wikipedia.
From ar.inspiredpencil.com
Endothermic Reaction Examples Everyday Life Endothermic Reaction Wikipedia For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. The opposite is an endothermic reaction, which usually takes up heat and is driven by an entropy increase in the system. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or. Endothermic Reaction Wikipedia.
From byjus.com
Difference Between Endothermic and Exothermic Reactions Chemistry Endothermic Reaction Wikipedia The opposite is an endothermic reaction, which usually takes up heat and is driven by an entropy increase in the system. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. In an endothermic reaction, the substance. Because heat is absorbed, endothermic. Revise and understand what endothermic and exothermic reactions. Endothermic Reaction Wikipedia.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Reaction Wikipedia For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their surroundings. An endothermic reaction is a chemical reaction that takes in energy from the surroundings. Because heat is absorbed,. Endothermic Reaction Wikipedia.
From www.slideserve.com
PPT Endothermic and Exothermic Reactions PowerPoint Presentation ID Endothermic Reaction Wikipedia An endothermic reaction is a chemical reaction that takes in energy from the surroundings. Because heat is absorbed, endothermic. The opposite of an endothermic reaction is an. The opposite is an endothermic reaction, which usually takes up heat and is driven by an entropy increase in the system. Revise and understand what endothermic and exothermic reactions are and how the. Endothermic Reaction Wikipedia.
From h-o-m-e.org
Endothermic Reactions The Science Behind Temperature Change Endothermic Reaction Wikipedia The opposite is an endothermic reaction, which usually takes up heat and is driven by an entropy increase in the system. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. The opposite of an endothermic reaction is an. An endothermic reaction is a reaction between two elements or compounds that absorbs heat energy. For an. Endothermic Reaction Wikipedia.
From resolutionsforyou.com
The Journey of an Endothermic Reaction Understanding the Reaction Endothermic Reaction Wikipedia For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. In an endothermic reaction, the substance. An endothermic reaction is a reaction between two elements or compounds that absorbs heat energy. The opposite is an endothermic reaction, which usually takes up heat and is driven by an entropy increase in. Endothermic Reaction Wikipedia.
From sciencenotes.org
Endothermic Reactions Definition and Examples Endothermic Reaction Wikipedia Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their surroundings. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. In an endothermic reaction, the substance. An endothermic reaction is a chemical reaction that absorbs thermal energy from. Endothermic Reaction Wikipedia.
From slideplayer.com
Endothermic and exothermic reactions ppt download Endothermic Reaction Wikipedia An endothermic reaction is a reaction between two elements or compounds that absorbs heat energy. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. An endothermic reaction is a chemical reaction that takes in energy from the surroundings. The opposite is an endothermic reaction, which usually takes up heat and is driven by an entropy. Endothermic Reaction Wikipedia.
From mmerevise.co.uk
Endothermic and Exothermic Reactions Revision MME Endothermic Reaction Wikipedia An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Because heat is absorbed, endothermic. An endothermic reaction is a reaction between two elements or compounds that absorbs heat energy. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their surroundings. For an endothermic chemical. Endothermic Reaction Wikipedia.
From www.thoughtco.com
Endothermic and Exothermic Chemical Reactions Endothermic Reaction Wikipedia The opposite of an endothermic reaction is an. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. The opposite is an endothermic reaction, which usually takes up heat and is driven by an entropy increase in the system. In an endothermic reaction, the substance. Revise and understand what endothermic and exothermic reactions are and how. Endothermic Reaction Wikipedia.
From www.thoughtco.com
Endothermic Reaction Examples Endothermic Reaction Wikipedia In an endothermic reaction, the substance. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. An endothermic reaction is a chemical reaction that takes in energy from the surroundings. The opposite of an endothermic reaction is an. Because heat is absorbed, endothermic. An endothermic reaction is a reaction between two elements or compounds that absorbs. Endothermic Reaction Wikipedia.
From www.slideserve.com
PPT Chapter 12 “Heat in Chemical Reactions” PowerPoint Presentation Endothermic Reaction Wikipedia An endothermic reaction is a chemical reaction that takes in energy from the surroundings. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their surroundings. An endothermic reaction is a reaction between two elements or compounds that absorbs heat energy. For an endothermic chemical reaction to proceed, the reactants. Endothermic Reaction Wikipedia.
From slideplayer.com
Exothermic and Endothermic Reactions ppt download Endothermic Reaction Wikipedia For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Because heat is absorbed, endothermic. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their. Endothermic Reaction Wikipedia.
From www.worksheetsplanet.com
What is an Endothermic Reaction Definition & Example Endothermic Reaction Wikipedia In an endothermic reaction, the substance. An endothermic reaction is a reaction between two elements or compounds that absorbs heat energy. The opposite of an endothermic reaction is an. The opposite is an endothermic reaction, which usually takes up heat and is driven by an entropy increase in the system. An endothermic reaction is a chemical reaction that takes in. Endothermic Reaction Wikipedia.
From gamesmartz.com
Endothermic Reaction Easy to Understand Endothermic Reaction Wikipedia For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. Because heat is absorbed, endothermic. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. In an endothermic reaction, the substance. The opposite of an endothermic reaction is an. The opposite is an endothermic reaction, which. Endothermic Reaction Wikipedia.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Reaction Wikipedia An endothermic reaction is a reaction between two elements or compounds that absorbs heat energy. In an endothermic reaction, the substance. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their surroundings. An endothermic reaction is a chemical reaction that takes in energy from the surroundings. An endothermic reaction. Endothermic Reaction Wikipedia.