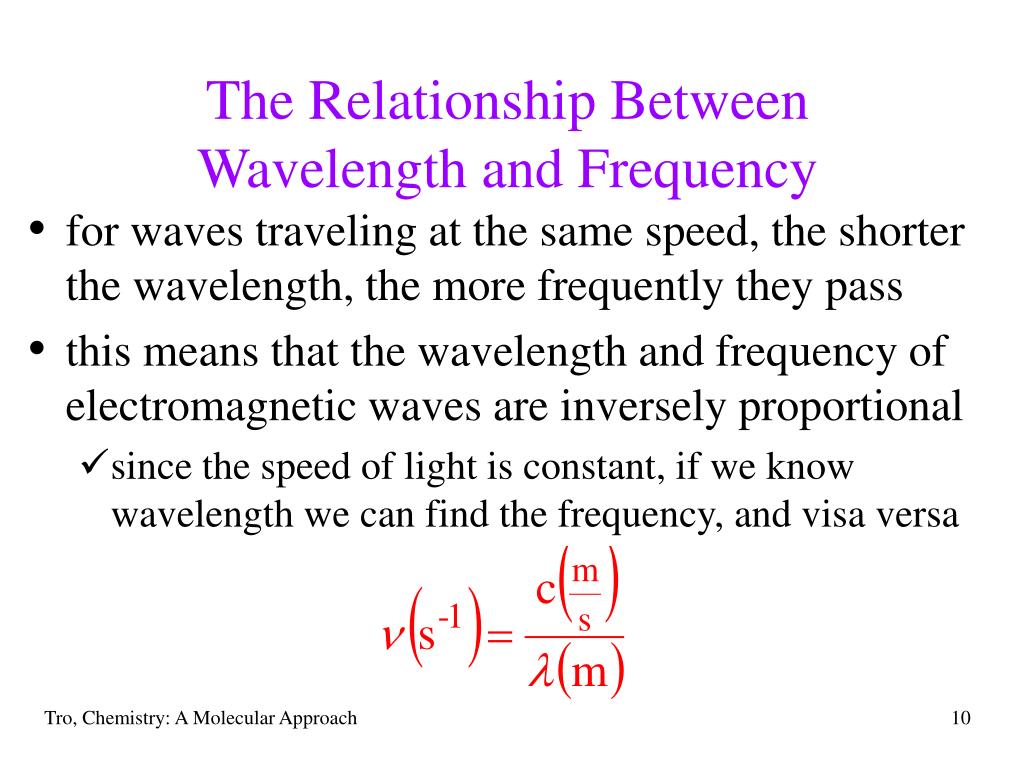
Frequency And Quantum Relationship . The relationship between frequency, wavelength, and velocity is expressed mathematically as f ·훌 = v. In this equation, f is frequency; Explain how the study of blackbody radiation lead to the understanding of quantized energy. The quantum mechanical proportionality between energy and frequency of a photon originates in planck's action quantum h, which has the dimension (energytime), i.e. Calculate the number of photons per second emitted by a monochromatic. Relate wavelength and frequency to the energy associated with. 훌 is lambda, the greek letter used. Define quantum states and their relationship to modern physics; Calculate the quantum energy of lights; Explain how photon energies vary across. Explain the relationship between the energy of a photon in joules or electron volts and its wavelength or frequency.
from www.slideserve.com
Explain how photon energies vary across. 훌 is lambda, the greek letter used. Explain how the study of blackbody radiation lead to the understanding of quantized energy. Relate wavelength and frequency to the energy associated with. Calculate the number of photons per second emitted by a monochromatic. In this equation, f is frequency; Explain the relationship between the energy of a photon in joules or electron volts and its wavelength or frequency. The quantum mechanical proportionality between energy and frequency of a photon originates in planck's action quantum h, which has the dimension (energytime), i.e. Calculate the quantum energy of lights; The relationship between frequency, wavelength, and velocity is expressed mathematically as f ·훌 = v.
PPT Chapter 7 The QuantumMechanical Model of the Atom PowerPoint
Frequency And Quantum Relationship Explain how the study of blackbody radiation lead to the understanding of quantized energy. Calculate the quantum energy of lights; The quantum mechanical proportionality between energy and frequency of a photon originates in planck's action quantum h, which has the dimension (energytime), i.e. 훌 is lambda, the greek letter used. Calculate the number of photons per second emitted by a monochromatic. The relationship between frequency, wavelength, and velocity is expressed mathematically as f ·훌 = v. Relate wavelength and frequency to the energy associated with. Explain how photon energies vary across. Define quantum states and their relationship to modern physics; Explain the relationship between the energy of a photon in joules or electron volts and its wavelength or frequency. In this equation, f is frequency; Explain how the study of blackbody radiation lead to the understanding of quantized energy.
From www.prosoundweb.com
A Group Of Frequencies The Relationship Between Time & Frequency Frequency And Quantum Relationship The relationship between frequency, wavelength, and velocity is expressed mathematically as f ·훌 = v. Explain the relationship between the energy of a photon in joules or electron volts and its wavelength or frequency. 훌 is lambda, the greek letter used. The quantum mechanical proportionality between energy and frequency of a photon originates in planck's action quantum h, which has. Frequency And Quantum Relationship.
From www.researchgate.net
Frequency spectrum for the quantum voltmeter in the subsampling mode Frequency And Quantum Relationship Define quantum states and their relationship to modern physics; Explain how photon energies vary across. 훌 is lambda, the greek letter used. Explain how the study of blackbody radiation lead to the understanding of quantized energy. Explain the relationship between the energy of a photon in joules or electron volts and its wavelength or frequency. Calculate the quantum energy of. Frequency And Quantum Relationship.
From www.youtube.com
Quantum Frequency Technologies Explained YouTube Frequency And Quantum Relationship Explain how photon energies vary across. Calculate the quantum energy of lights; Explain the relationship between the energy of a photon in joules or electron volts and its wavelength or frequency. Define quantum states and their relationship to modern physics; The quantum mechanical proportionality between energy and frequency of a photon originates in planck's action quantum h, which has the. Frequency And Quantum Relationship.
From animalia-life.club
Wavelength And Frequency Diagram Frequency And Quantum Relationship The relationship between frequency, wavelength, and velocity is expressed mathematically as f ·훌 = v. Explain how photon energies vary across. 훌 is lambda, the greek letter used. Calculate the quantum energy of lights; Explain how the study of blackbody radiation lead to the understanding of quantized energy. Explain the relationship between the energy of a photon in joules or. Frequency And Quantum Relationship.
From www.youtube.com
Relationship Between Wavelength, Frequency, and Energy (Direct or Frequency And Quantum Relationship Explain the relationship between the energy of a photon in joules or electron volts and its wavelength or frequency. Calculate the number of photons per second emitted by a monochromatic. 훌 is lambda, the greek letter used. Calculate the quantum energy of lights; Explain how photon energies vary across. In this equation, f is frequency; The relationship between frequency, wavelength,. Frequency And Quantum Relationship.
From www.researchgate.net
(PDF) Generation of multiphoton entangled quantum states by means of Frequency And Quantum Relationship Relate wavelength and frequency to the energy associated with. Explain the relationship between the energy of a photon in joules or electron volts and its wavelength or frequency. Define quantum states and their relationship to modern physics; Explain how photon energies vary across. Calculate the number of photons per second emitted by a monochromatic. 훌 is lambda, the greek letter. Frequency And Quantum Relationship.
From www.researchgate.net
Quantum oscillations in the PDO resonance frequency at different angles Frequency And Quantum Relationship Define quantum states and their relationship to modern physics; Explain how the study of blackbody radiation lead to the understanding of quantized energy. The quantum mechanical proportionality between energy and frequency of a photon originates in planck's action quantum h, which has the dimension (energytime), i.e. Calculate the quantum energy of lights; Calculate the number of photons per second emitted. Frequency And Quantum Relationship.
From www.researchgate.net
(PDF) The Wave Model of Light vs. the Quantum Model The Relationship Frequency And Quantum Relationship 훌 is lambda, the greek letter used. Calculate the number of photons per second emitted by a monochromatic. Explain how photon energies vary across. The quantum mechanical proportionality between energy and frequency of a photon originates in planck's action quantum h, which has the dimension (energytime), i.e. Calculate the quantum energy of lights; In this equation, f is frequency; Define. Frequency And Quantum Relationship.
From www.researchgate.net
Measured singlephoton spectrum of the integrated quantum frequency Frequency And Quantum Relationship In this equation, f is frequency; Explain how the study of blackbody radiation lead to the understanding of quantized energy. Calculate the number of photons per second emitted by a monochromatic. Define quantum states and their relationship to modern physics; Relate wavelength and frequency to the energy associated with. 훌 is lambda, the greek letter used. Explain the relationship between. Frequency And Quantum Relationship.
From www.researchgate.net
Angular dependence of quantum oscillation frequency and cyclotron mass Frequency And Quantum Relationship Explain how the study of blackbody radiation lead to the understanding of quantized energy. Calculate the quantum energy of lights; Define quantum states and their relationship to modern physics; Explain how photon energies vary across. Explain the relationship between the energy of a photon in joules or electron volts and its wavelength or frequency. The relationship between frequency, wavelength, and. Frequency And Quantum Relationship.
From www.researchgate.net
Quantum state characterization of photon pairs in the frequency Frequency And Quantum Relationship Explain how the study of blackbody radiation lead to the understanding of quantized energy. Explain the relationship between the energy of a photon in joules or electron volts and its wavelength or frequency. Calculate the number of photons per second emitted by a monochromatic. In this equation, f is frequency; The relationship between frequency, wavelength, and velocity is expressed mathematically. Frequency And Quantum Relationship.
From www.researchgate.net
Quantum entanglement between frequency and position space as function Frequency And Quantum Relationship Define quantum states and their relationship to modern physics; Calculate the quantum energy of lights; Relate wavelength and frequency to the energy associated with. Explain how the study of blackbody radiation lead to the understanding of quantized energy. In this equation, f is frequency; The quantum mechanical proportionality between energy and frequency of a photon originates in planck's action quantum. Frequency And Quantum Relationship.
From www.researchgate.net
Rotationfrequency dependence of a quantum vortex state formation Frequency And Quantum Relationship Define quantum states and their relationship to modern physics; Explain the relationship between the energy of a photon in joules or electron volts and its wavelength or frequency. Explain how photon energies vary across. 훌 is lambda, the greek letter used. Relate wavelength and frequency to the energy associated with. The quantum mechanical proportionality between energy and frequency of a. Frequency And Quantum Relationship.
From www.simscale.com
What Is Modal Analysis and Why Is It Necessary? SimScale Blog Frequency And Quantum Relationship Explain how photon energies vary across. Explain the relationship between the energy of a photon in joules or electron volts and its wavelength or frequency. Explain how the study of blackbody radiation lead to the understanding of quantized energy. Relate wavelength and frequency to the energy associated with. 훌 is lambda, the greek letter used. Calculate the quantum energy of. Frequency And Quantum Relationship.
From www.researchgate.net
the optical quantum frequency as a function R b k z Download Frequency And Quantum Relationship The quantum mechanical proportionality between energy and frequency of a photon originates in planck's action quantum h, which has the dimension (energytime), i.e. Define quantum states and their relationship to modern physics; Explain how photon energies vary across. 훌 is lambda, the greek letter used. In this equation, f is frequency; Calculate the quantum energy of lights; Relate wavelength and. Frequency And Quantum Relationship.
From www.forbes.com
The Science Of Agents Of S.H.I.E.L.D. What Is Quantum Harmonic Frequency And Quantum Relationship Explain how photon energies vary across. The relationship between frequency, wavelength, and velocity is expressed mathematically as f ·훌 = v. Relate wavelength and frequency to the energy associated with. The quantum mechanical proportionality between energy and frequency of a photon originates in planck's action quantum h, which has the dimension (energytime), i.e. In this equation, f is frequency; Calculate. Frequency And Quantum Relationship.
From www.researchgate.net
Principles of quantum frequency conversion. a. Frequency conversion Frequency And Quantum Relationship Explain how the study of blackbody radiation lead to the understanding of quantized energy. The quantum mechanical proportionality between energy and frequency of a photon originates in planck's action quantum h, which has the dimension (energytime), i.e. Explain how photon energies vary across. Define quantum states and their relationship to modern physics; Explain the relationship between the energy of a. Frequency And Quantum Relationship.
From nlo.stanford.edu
Devices for Quantum Frequency Conversion Fejer Group Frequency And Quantum Relationship The quantum mechanical proportionality between energy and frequency of a photon originates in planck's action quantum h, which has the dimension (energytime), i.e. In this equation, f is frequency; Explain how photon energies vary across. Calculate the number of photons per second emitted by a monochromatic. Explain how the study of blackbody radiation lead to the understanding of quantized energy.. Frequency And Quantum Relationship.
From www.thednacompany.com
Quantum Biology The Symbiotic Relationship Between Frequencies and He Frequency And Quantum Relationship Explain how photon energies vary across. Explain how the study of blackbody radiation lead to the understanding of quantized energy. Calculate the number of photons per second emitted by a monochromatic. Define quantum states and their relationship to modern physics; Calculate the quantum energy of lights; 훌 is lambda, the greek letter used. In this equation, f is frequency; Explain. Frequency And Quantum Relationship.
From www.researchgate.net
Conceptual diagram of the frequency spectrum for multimode quantum Frequency And Quantum Relationship Define quantum states and their relationship to modern physics; In this equation, f is frequency; The quantum mechanical proportionality between energy and frequency of a photon originates in planck's action quantum h, which has the dimension (energytime), i.e. The relationship between frequency, wavelength, and velocity is expressed mathematically as f ·훌 = v. Explain how the study of blackbody radiation. Frequency And Quantum Relationship.
From www.sprawls.org
Energy and Radiation Frequency And Quantum Relationship In this equation, f is frequency; Explain how the study of blackbody radiation lead to the understanding of quantized energy. Calculate the number of photons per second emitted by a monochromatic. The relationship between frequency, wavelength, and velocity is expressed mathematically as f ·훌 = v. Explain how photon energies vary across. Calculate the quantum energy of lights; Define quantum. Frequency And Quantum Relationship.
From www.researchgate.net
Frequency offset heterodyne readout a Principle of the readout scheme Frequency And Quantum Relationship Calculate the number of photons per second emitted by a monochromatic. Define quantum states and their relationship to modern physics; Relate wavelength and frequency to the energy associated with. The relationship between frequency, wavelength, and velocity is expressed mathematically as f ·훌 = v. 훌 is lambda, the greek letter used. In this equation, f is frequency; Calculate the quantum. Frequency And Quantum Relationship.
From studylib.net
Planck's Equation E = hv Frequency And Quantum Relationship Explain how photon energies vary across. Explain how the study of blackbody radiation lead to the understanding of quantized energy. Calculate the quantum energy of lights; The relationship between frequency, wavelength, and velocity is expressed mathematically as f ·훌 = v. Calculate the number of photons per second emitted by a monochromatic. In this equation, f is frequency; Explain the. Frequency And Quantum Relationship.
From www.youtube.com
Photon Energy and the Planck Constant IB Physics YouTube Frequency And Quantum Relationship Define quantum states and their relationship to modern physics; Explain how the study of blackbody radiation lead to the understanding of quantized energy. The relationship between frequency, wavelength, and velocity is expressed mathematically as f ·훌 = v. Relate wavelength and frequency to the energy associated with. Calculate the number of photons per second emitted by a monochromatic. In this. Frequency And Quantum Relationship.
From www.researchgate.net
(a) Quantum frequency mixing schematic. The effective Hamiltonian (red Frequency And Quantum Relationship Calculate the number of photons per second emitted by a monochromatic. Relate wavelength and frequency to the energy associated with. 훌 is lambda, the greek letter used. The quantum mechanical proportionality between energy and frequency of a photon originates in planck's action quantum h, which has the dimension (energytime), i.e. Explain how the study of blackbody radiation lead to the. Frequency And Quantum Relationship.
From www.researchgate.net
Frequency analysis of the `giant quantum oscillations' in ultrasound Frequency And Quantum Relationship Explain how the study of blackbody radiation lead to the understanding of quantized energy. Calculate the number of photons per second emitted by a monochromatic. Relate wavelength and frequency to the energy associated with. Calculate the quantum energy of lights; Explain how photon energies vary across. The quantum mechanical proportionality between energy and frequency of a photon originates in planck's. Frequency And Quantum Relationship.
From www.slideserve.com
PPT Chapter 7 The QuantumMechanical Model of the Atom PowerPoint Frequency And Quantum Relationship 훌 is lambda, the greek letter used. The relationship between frequency, wavelength, and velocity is expressed mathematically as f ·훌 = v. Calculate the number of photons per second emitted by a monochromatic. Relate wavelength and frequency to the energy associated with. Calculate the quantum energy of lights; The quantum mechanical proportionality between energy and frequency of a photon originates. Frequency And Quantum Relationship.
From www.optica-opn.org
Optics & Photonics News Quantum Information Processing in the Frequency And Quantum Relationship Relate wavelength and frequency to the energy associated with. 훌 is lambda, the greek letter used. Explain the relationship between the energy of a photon in joules or electron volts and its wavelength or frequency. In this equation, f is frequency; Calculate the number of photons per second emitted by a monochromatic. Explain how photon energies vary across. Calculate the. Frequency And Quantum Relationship.
From www.researchgate.net
Quantum entanglement between frequency and position space as function Frequency And Quantum Relationship Explain how photon energies vary across. In this equation, f is frequency; 훌 is lambda, the greek letter used. Define quantum states and their relationship to modern physics; Explain the relationship between the energy of a photon in joules or electron volts and its wavelength or frequency. The relationship between frequency, wavelength, and velocity is expressed mathematically as f ·훌. Frequency And Quantum Relationship.
From chem.libretexts.org
1 The Rise of Quantum Mechanics (Lecture) Chemistry LibreTexts Frequency And Quantum Relationship Define quantum states and their relationship to modern physics; Calculate the number of photons per second emitted by a monochromatic. Explain how photon energies vary across. In this equation, f is frequency; Relate wavelength and frequency to the energy associated with. The relationship between frequency, wavelength, and velocity is expressed mathematically as f ·훌 = v. Explain how the study. Frequency And Quantum Relationship.
From www.slideserve.com
PPT Photochemistry Ozone Formation and Depletion PowerPoint Frequency And Quantum Relationship Explain how photon energies vary across. Relate wavelength and frequency to the energy associated with. 훌 is lambda, the greek letter used. The quantum mechanical proportionality between energy and frequency of a photon originates in planck's action quantum h, which has the dimension (energytime), i.e. Calculate the quantum energy of lights; Explain how the study of blackbody radiation lead to. Frequency And Quantum Relationship.
From slidetodoc.com
Quantum Qualities frequency wavelength and momentum of photons Frequency And Quantum Relationship Calculate the quantum energy of lights; 훌 is lambda, the greek letter used. Explain how the study of blackbody radiation lead to the understanding of quantized energy. The quantum mechanical proportionality between energy and frequency of a photon originates in planck's action quantum h, which has the dimension (energytime), i.e. The relationship between frequency, wavelength, and velocity is expressed mathematically. Frequency And Quantum Relationship.
From www.researchgate.net
Principles of quantum frequency conversion. a. Frequency conversion Frequency And Quantum Relationship 훌 is lambda, the greek letter used. Explain the relationship between the energy of a photon in joules or electron volts and its wavelength or frequency. Define quantum states and their relationship to modern physics; Relate wavelength and frequency to the energy associated with. Calculate the quantum energy of lights; Explain how photon energies vary across. The relationship between frequency,. Frequency And Quantum Relationship.
From courses.lumenlearning.com
3.1 Energy General College Chemistry I Frequency And Quantum Relationship The relationship between frequency, wavelength, and velocity is expressed mathematically as f ·훌 = v. Explain how the study of blackbody radiation lead to the understanding of quantized energy. 훌 is lambda, the greek letter used. Define quantum states and their relationship to modern physics; Calculate the quantum energy of lights; Explain the relationship between the energy of a photon. Frequency And Quantum Relationship.
From www.researchgate.net
Quantum frequency correlations of highdimensional BFCs. a Experimental Frequency And Quantum Relationship 훌 is lambda, the greek letter used. Relate wavelength and frequency to the energy associated with. Explain how photon energies vary across. Explain how the study of blackbody radiation lead to the understanding of quantized energy. Calculate the quantum energy of lights; Define quantum states and their relationship to modern physics; In this equation, f is frequency; The relationship between. Frequency And Quantum Relationship.