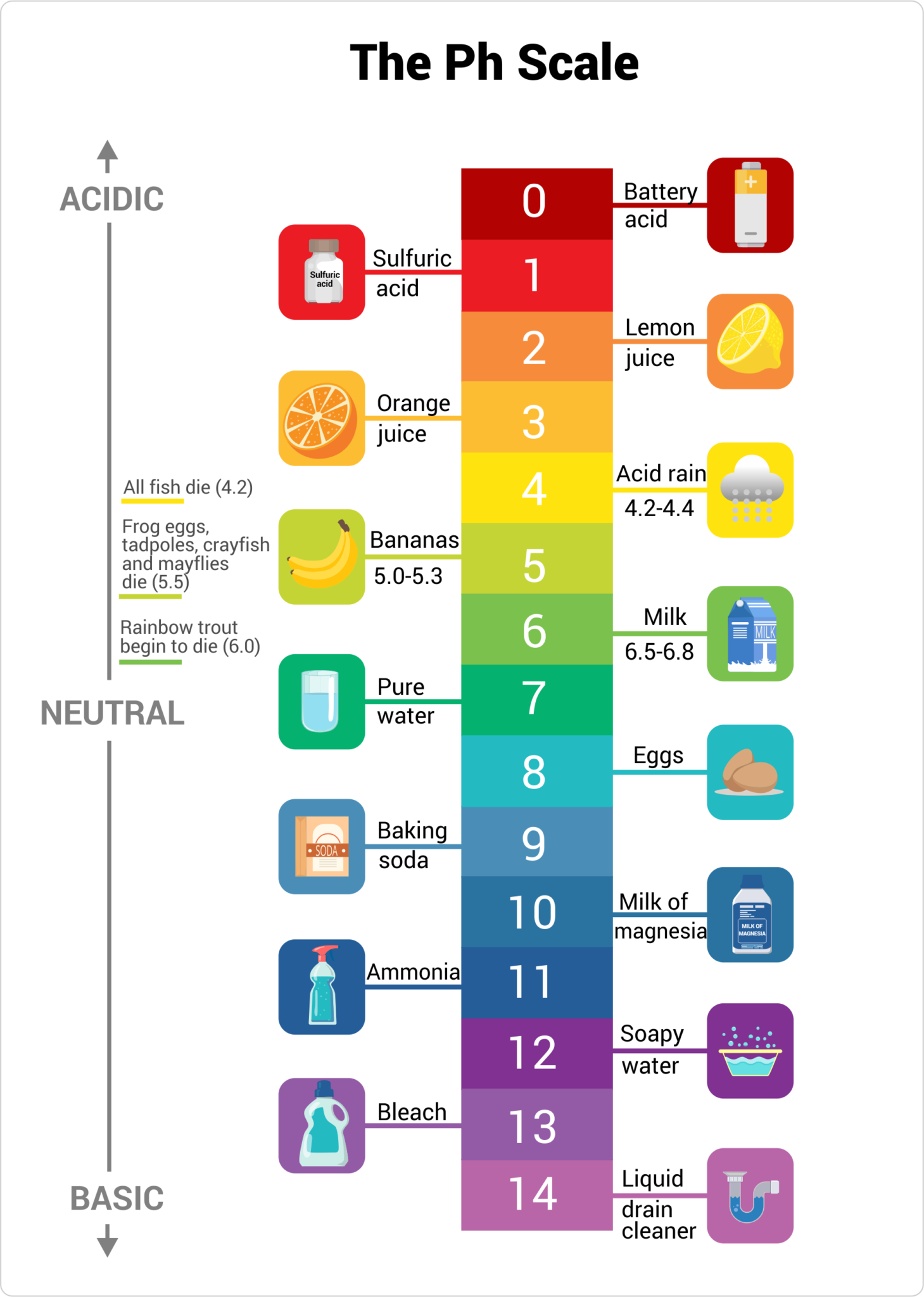
What Is The Ph Value Of Ammonia . By monitoring and controlling the ph level of ammonia solutions, the desired properties and effects can be achieved effectively. Calculate ph of ammonia by using dissociation constant (k b) value of ammonia. A 1% solution of ammonia in water has a ph of around 11.6, indicating its basic nature. 34 rows the scale runs from 0 (most acidic) to 14 (most alkaline or basic), where 7 is neutral ph. Chemicals with ph values from 0 up to 7 are acids, those with a ph value of 7 are. The ph of standard ammonia is about 11. The ph value of a solution is a crucial factor in determining the toxicity and behavior of ammonia, a common water. The ph of ammonia in water can be. It can convert ph to h +, as well as calculate ph from the ionization constant and concentration. With this ph calculator, you can determine the ph of a solution in a few ways.
from www.mometrix.com
Chemicals with ph values from 0 up to 7 are acids, those with a ph value of 7 are. The ph value of a solution is a crucial factor in determining the toxicity and behavior of ammonia, a common water. It can convert ph to h +, as well as calculate ph from the ionization constant and concentration. 34 rows the scale runs from 0 (most acidic) to 14 (most alkaline or basic), where 7 is neutral ph. By monitoring and controlling the ph level of ammonia solutions, the desired properties and effects can be achieved effectively. The ph of standard ammonia is about 11. With this ph calculator, you can determine the ph of a solution in a few ways. Calculate ph of ammonia by using dissociation constant (k b) value of ammonia. A 1% solution of ammonia in water has a ph of around 11.6, indicating its basic nature. The ph of ammonia in water can be.
pH Overview (Chemistry Review Video)
What Is The Ph Value Of Ammonia The ph value of a solution is a crucial factor in determining the toxicity and behavior of ammonia, a common water. It can convert ph to h +, as well as calculate ph from the ionization constant and concentration. A 1% solution of ammonia in water has a ph of around 11.6, indicating its basic nature. With this ph calculator, you can determine the ph of a solution in a few ways. The ph of standard ammonia is about 11. Calculate ph of ammonia by using dissociation constant (k b) value of ammonia. The ph value of a solution is a crucial factor in determining the toxicity and behavior of ammonia, a common water. The ph of ammonia in water can be. 34 rows the scale runs from 0 (most acidic) to 14 (most alkaline or basic), where 7 is neutral ph. Chemicals with ph values from 0 up to 7 are acids, those with a ph value of 7 are. By monitoring and controlling the ph level of ammonia solutions, the desired properties and effects can be achieved effectively.
From solvedlib.com
What is the pH of a 1.5 M aqueous ammonia solution at… SolvedLib What Is The Ph Value Of Ammonia A 1% solution of ammonia in water has a ph of around 11.6, indicating its basic nature. The ph value of a solution is a crucial factor in determining the toxicity and behavior of ammonia, a common water. By monitoring and controlling the ph level of ammonia solutions, the desired properties and effects can be achieved effectively. The ph of. What Is The Ph Value Of Ammonia.
From freddyzebcarney.blogspot.com
Is Ammonia Acidic or Alkaline FreddyzebCarney What Is The Ph Value Of Ammonia The ph of standard ammonia is about 11. The ph value of a solution is a crucial factor in determining the toxicity and behavior of ammonia, a common water. With this ph calculator, you can determine the ph of a solution in a few ways. 34 rows the scale runs from 0 (most acidic) to 14 (most alkaline or basic),. What Is The Ph Value Of Ammonia.
From www.vrogue.co
The Ph Scale Of Common Chemicals Chemistry Chemistry vrogue.co What Is The Ph Value Of Ammonia By monitoring and controlling the ph level of ammonia solutions, the desired properties and effects can be achieved effectively. It can convert ph to h +, as well as calculate ph from the ionization constant and concentration. The ph value of a solution is a crucial factor in determining the toxicity and behavior of ammonia, a common water. Calculate ph. What Is The Ph Value Of Ammonia.
From mavink.com
Ph Diagram For Ammonia What Is The Ph Value Of Ammonia The ph of ammonia in water can be. Chemicals with ph values from 0 up to 7 are acids, those with a ph value of 7 are. With this ph calculator, you can determine the ph of a solution in a few ways. Calculate ph of ammonia by using dissociation constant (k b) value of ammonia. By monitoring and controlling. What Is The Ph Value Of Ammonia.
From sciencenotes.org
The pH Scale of Common Chemicals What Is The Ph Value Of Ammonia A 1% solution of ammonia in water has a ph of around 11.6, indicating its basic nature. The ph value of a solution is a crucial factor in determining the toxicity and behavior of ammonia, a common water. With this ph calculator, you can determine the ph of a solution in a few ways. The ph of ammonia in water. What Is The Ph Value Of Ammonia.
From blueridgekoi.com
pH and Ammonia What You Might Not Know Blue Ridge Fish Hatchery What Is The Ph Value Of Ammonia It can convert ph to h +, as well as calculate ph from the ionization constant and concentration. Calculate ph of ammonia by using dissociation constant (k b) value of ammonia. 34 rows the scale runs from 0 (most acidic) to 14 (most alkaline or basic), where 7 is neutral ph. By monitoring and controlling the ph level of ammonia. What Is The Ph Value Of Ammonia.
From mungfali.com
Ammonia Ph Chart What Is The Ph Value Of Ammonia 34 rows the scale runs from 0 (most acidic) to 14 (most alkaline or basic), where 7 is neutral ph. By monitoring and controlling the ph level of ammonia solutions, the desired properties and effects can be achieved effectively. A 1% solution of ammonia in water has a ph of around 11.6, indicating its basic nature. The ph of standard. What Is The Ph Value Of Ammonia.
From www.researchgate.net
Color and pH change of standard curve of ammonia in fast urea solution What Is The Ph Value Of Ammonia It can convert ph to h +, as well as calculate ph from the ionization constant and concentration. By monitoring and controlling the ph level of ammonia solutions, the desired properties and effects can be achieved effectively. A 1% solution of ammonia in water has a ph of around 11.6, indicating its basic nature. 34 rows the scale runs from. What Is The Ph Value Of Ammonia.
From www.youtube.com
pH of Household Ammonia (NH3) and Color Changing pH Paper YouTube What Is The Ph Value Of Ammonia The ph of ammonia in water can be. Chemicals with ph values from 0 up to 7 are acids, those with a ph value of 7 are. Calculate ph of ammonia by using dissociation constant (k b) value of ammonia. The ph value of a solution is a crucial factor in determining the toxicity and behavior of ammonia, a common. What Is The Ph Value Of Ammonia.
From www.expii.com
What Is pH? — Definition & Overview Expii What Is The Ph Value Of Ammonia A 1% solution of ammonia in water has a ph of around 11.6, indicating its basic nature. 34 rows the scale runs from 0 (most acidic) to 14 (most alkaline or basic), where 7 is neutral ph. Calculate ph of ammonia by using dissociation constant (k b) value of ammonia. By monitoring and controlling the ph level of ammonia solutions,. What Is The Ph Value Of Ammonia.
From www.slideserve.com
PPT Lectures 67 Production of ammonia PowerPoint Presentation, free What Is The Ph Value Of Ammonia By monitoring and controlling the ph level of ammonia solutions, the desired properties and effects can be achieved effectively. The ph of ammonia in water can be. Calculate ph of ammonia by using dissociation constant (k b) value of ammonia. Chemicals with ph values from 0 up to 7 are acids, those with a ph value of 7 are. The. What Is The Ph Value Of Ammonia.
From www.researchgate.net
1. Ammonia gas partitioning to water and resulting pH. Download Table What Is The Ph Value Of Ammonia 34 rows the scale runs from 0 (most acidic) to 14 (most alkaline or basic), where 7 is neutral ph. The ph of ammonia in water can be. The ph value of a solution is a crucial factor in determining the toxicity and behavior of ammonia, a common water. Calculate ph of ammonia by using dissociation constant (k b) value. What Is The Ph Value Of Ammonia.
From www.mankysanke.co.uk
Ammonia What Is The Ph Value Of Ammonia The ph of standard ammonia is about 11. Chemicals with ph values from 0 up to 7 are acids, those with a ph value of 7 are. With this ph calculator, you can determine the ph of a solution in a few ways. The ph of ammonia in water can be. A 1% solution of ammonia in water has a. What Is The Ph Value Of Ammonia.
From mungfali.com
Ammonia On PH Scale What Is The Ph Value Of Ammonia Chemicals with ph values from 0 up to 7 are acids, those with a ph value of 7 are. The ph of standard ammonia is about 11. The ph value of a solution is a crucial factor in determining the toxicity and behavior of ammonia, a common water. A 1% solution of ammonia in water has a ph of around. What Is The Ph Value Of Ammonia.
From ecologywa.blogspot.com
ECOconnect Let’s Talk Science A pH Solution What Is The Ph Value Of Ammonia With this ph calculator, you can determine the ph of a solution in a few ways. By monitoring and controlling the ph level of ammonia solutions, the desired properties and effects can be achieved effectively. A 1% solution of ammonia in water has a ph of around 11.6, indicating its basic nature. The ph of ammonia in water can be.. What Is The Ph Value Of Ammonia.
From www.researchgate.net
Comparison of pH for ammonia, carbon dioxide and pure water. Download What Is The Ph Value Of Ammonia By monitoring and controlling the ph level of ammonia solutions, the desired properties and effects can be achieved effectively. The ph of ammonia in water can be. A 1% solution of ammonia in water has a ph of around 11.6, indicating its basic nature. The ph of standard ammonia is about 11. With this ph calculator, you can determine the. What Is The Ph Value Of Ammonia.
From askfilo.com
An ammonia ammonium chloride buffer has a pH value of 9 with [NH3 ]=0.25.. What Is The Ph Value Of Ammonia The ph of ammonia in water can be. A 1% solution of ammonia in water has a ph of around 11.6, indicating its basic nature. Calculate ph of ammonia by using dissociation constant (k b) value of ammonia. With this ph calculator, you can determine the ph of a solution in a few ways. 34 rows the scale runs from. What Is The Ph Value Of Ammonia.
From www.careerpower.in
pH Values List for Common Substances What Is The Ph Value Of Ammonia With this ph calculator, you can determine the ph of a solution in a few ways. The ph of ammonia in water can be. 34 rows the scale runs from 0 (most acidic) to 14 (most alkaline or basic), where 7 is neutral ph. It can convert ph to h +, as well as calculate ph from the ionization constant. What Is The Ph Value Of Ammonia.
From bertigamas.github.io
Nh3 Ph Sinau What Is The Ph Value Of Ammonia With this ph calculator, you can determine the ph of a solution in a few ways. The ph value of a solution is a crucial factor in determining the toxicity and behavior of ammonia, a common water. A 1% solution of ammonia in water has a ph of around 11.6, indicating its basic nature. Calculate ph of ammonia by using. What Is The Ph Value Of Ammonia.
From www.researchgate.net
pH values, ammonia concentration, and pool of individual and total SCFA What Is The Ph Value Of Ammonia The ph of ammonia in water can be. The ph of standard ammonia is about 11. The ph value of a solution is a crucial factor in determining the toxicity and behavior of ammonia, a common water. It can convert ph to h +, as well as calculate ph from the ionization constant and concentration. Chemicals with ph values from. What Is The Ph Value Of Ammonia.
From www.researchgate.net
The pH of the ammonia, morpholine and ethanolamine solutions and the pH What Is The Ph Value Of Ammonia 34 rows the scale runs from 0 (most acidic) to 14 (most alkaline or basic), where 7 is neutral ph. The ph of ammonia in water can be. A 1% solution of ammonia in water has a ph of around 11.6, indicating its basic nature. By monitoring and controlling the ph level of ammonia solutions, the desired properties and effects. What Is The Ph Value Of Ammonia.
From www.mometrix.com
pH Overview (Chemistry Review Video) What Is The Ph Value Of Ammonia It can convert ph to h +, as well as calculate ph from the ionization constant and concentration. The ph of ammonia in water can be. Calculate ph of ammonia by using dissociation constant (k b) value of ammonia. The ph value of a solution is a crucial factor in determining the toxicity and behavior of ammonia, a common water.. What Is The Ph Value Of Ammonia.
From www.researchgate.net
Percent abundance of ammonia and ammonium across a range of pH values What Is The Ph Value Of Ammonia Chemicals with ph values from 0 up to 7 are acids, those with a ph value of 7 are. With this ph calculator, you can determine the ph of a solution in a few ways. The ph value of a solution is a crucial factor in determining the toxicity and behavior of ammonia, a common water. 34 rows the scale. What Is The Ph Value Of Ammonia.
From www.researchgate.net
Effect of temperature and pH on the fraction of unionized ammonia (NH 3 What Is The Ph Value Of Ammonia It can convert ph to h +, as well as calculate ph from the ionization constant and concentration. Calculate ph of ammonia by using dissociation constant (k b) value of ammonia. 34 rows the scale runs from 0 (most acidic) to 14 (most alkaline or basic), where 7 is neutral ph. A 1% solution of ammonia in water has a. What Is The Ph Value Of Ammonia.
From www.oist.jp
pH Values of Common Substances Okinawa Institute of Science and What Is The Ph Value Of Ammonia It can convert ph to h +, as well as calculate ph from the ionization constant and concentration. A 1% solution of ammonia in water has a ph of around 11.6, indicating its basic nature. Calculate ph of ammonia by using dissociation constant (k b) value of ammonia. The ph of standard ammonia is about 11. With this ph calculator,. What Is The Ph Value Of Ammonia.
From www.researchgate.net
Equilibrium fraction of total ammonia, nitric acid, and hydrochloric What Is The Ph Value Of Ammonia The ph of standard ammonia is about 11. The ph value of a solution is a crucial factor in determining the toxicity and behavior of ammonia, a common water. 34 rows the scale runs from 0 (most acidic) to 14 (most alkaline or basic), where 7 is neutral ph. A 1% solution of ammonia in water has a ph of. What Is The Ph Value Of Ammonia.
From www.freepik.com
Premium Vector Ammonium versus ammonia comparison science vector What Is The Ph Value Of Ammonia A 1% solution of ammonia in water has a ph of around 11.6, indicating its basic nature. The ph of standard ammonia is about 11. Calculate ph of ammonia by using dissociation constant (k b) value of ammonia. 34 rows the scale runs from 0 (most acidic) to 14 (most alkaline or basic), where 7 is neutral ph. The ph. What Is The Ph Value Of Ammonia.
From www.coursehero.com
[Solved] Base 1) The pH of a solution of 0.061 mol/L ammonia is ab.cd What Is The Ph Value Of Ammonia By monitoring and controlling the ph level of ammonia solutions, the desired properties and effects can be achieved effectively. 34 rows the scale runs from 0 (most acidic) to 14 (most alkaline or basic), where 7 is neutral ph. It can convert ph to h +, as well as calculate ph from the ionization constant and concentration. The ph of. What Is The Ph Value Of Ammonia.
From www.chegg.com
Calculate the ratio (NH3]/[NH4+) in ammonia/ammonium What Is The Ph Value Of Ammonia Chemicals with ph values from 0 up to 7 are acids, those with a ph value of 7 are. The ph of standard ammonia is about 11. By monitoring and controlling the ph level of ammonia solutions, the desired properties and effects can be achieved effectively. Calculate ph of ammonia by using dissociation constant (k b) value of ammonia. The. What Is The Ph Value Of Ammonia.
From www.chegg.com
Solved 25) The pH of a 0.25 M aqueous solution ammonia, NH3, What Is The Ph Value Of Ammonia A 1% solution of ammonia in water has a ph of around 11.6, indicating its basic nature. The ph value of a solution is a crucial factor in determining the toxicity and behavior of ammonia, a common water. The ph of standard ammonia is about 11. It can convert ph to h +, as well as calculate ph from the. What Is The Ph Value Of Ammonia.
From www.mankysanke.co.uk
Ammonia in show vats What Is The Ph Value Of Ammonia Calculate ph of ammonia by using dissociation constant (k b) value of ammonia. With this ph calculator, you can determine the ph of a solution in a few ways. It can convert ph to h +, as well as calculate ph from the ionization constant and concentration. The ph value of a solution is a crucial factor in determining the. What Is The Ph Value Of Ammonia.
From www.youtube.com
calculating pH of ammonia YouTube What Is The Ph Value Of Ammonia The ph value of a solution is a crucial factor in determining the toxicity and behavior of ammonia, a common water. The ph of ammonia in water can be. Calculate ph of ammonia by using dissociation constant (k b) value of ammonia. 34 rows the scale runs from 0 (most acidic) to 14 (most alkaline or basic), where 7 is. What Is The Ph Value Of Ammonia.
From www.researchgate.net
(A) Correlation between pH and the ratio between ammonia and ammonium What Is The Ph Value Of Ammonia The ph value of a solution is a crucial factor in determining the toxicity and behavior of ammonia, a common water. It can convert ph to h +, as well as calculate ph from the ionization constant and concentration. A 1% solution of ammonia in water has a ph of around 11.6, indicating its basic nature. Chemicals with ph values. What Is The Ph Value Of Ammonia.
From mavink.com
Ammonia Ph Diagram What Is The Ph Value Of Ammonia The ph of standard ammonia is about 11. A 1% solution of ammonia in water has a ph of around 11.6, indicating its basic nature. The ph of ammonia in water can be. The ph value of a solution is a crucial factor in determining the toxicity and behavior of ammonia, a common water. Chemicals with ph values from 0. What Is The Ph Value Of Ammonia.
From www.researchgate.net
Effects of pH and temperature on the distribution of ammonia and What Is The Ph Value Of Ammonia The ph value of a solution is a crucial factor in determining the toxicity and behavior of ammonia, a common water. A 1% solution of ammonia in water has a ph of around 11.6, indicating its basic nature. The ph of standard ammonia is about 11. With this ph calculator, you can determine the ph of a solution in a. What Is The Ph Value Of Ammonia.