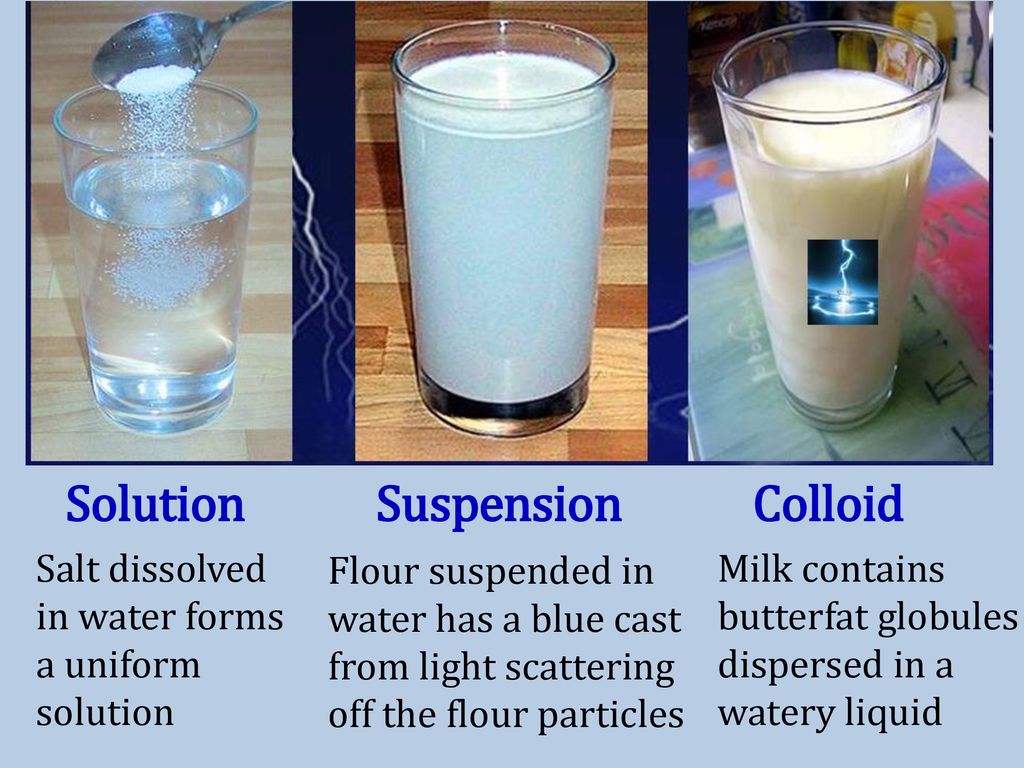
Uniform Solution Example . Solutions are homogeneous mixtures of two or more substances whose components are uniformly distributed on a microscopic scale. A solution is a homogeneous mixture comprising smaller component/s called solute/s of small molecules or ions comparable in size to the molecules of a larger. Solutions exist for every possible phase of the solute and the solvent. Salt water, for example, is a solution of solid \(\ce{nacl}\) in liquid water,. Single substance that does the dissolving substance present in the largest amount. Homogeneous mixtures, also known as solutions, are uniform throughout. Homogeneous solutions are solutions with uniform composition and properties throughout the solution. In a solution, the particles of the substances are evenly distributed, resulting in a single phase. Trail mix, salad, and blood (which is also called a suspension) are examples of heterogenous mixtures. 1 or more substance that is. For example a cup of.
from slideplayer.com
Salt water, for example, is a solution of solid \(\ce{nacl}\) in liquid water,. In a solution, the particles of the substances are evenly distributed, resulting in a single phase. Single substance that does the dissolving substance present in the largest amount. For example a cup of. Solutions are homogeneous mixtures of two or more substances whose components are uniformly distributed on a microscopic scale. 1 or more substance that is. Solutions exist for every possible phase of the solute and the solvent. Homogeneous solutions are solutions with uniform composition and properties throughout the solution. Trail mix, salad, and blood (which is also called a suspension) are examples of heterogenous mixtures. A solution is a homogeneous mixture comprising smaller component/s called solute/s of small molecules or ions comparable in size to the molecules of a larger.
Solutions, Colloids, and Suspensions ppt download
Uniform Solution Example Homogeneous mixtures, also known as solutions, are uniform throughout. Salt water, for example, is a solution of solid \(\ce{nacl}\) in liquid water,. Solutions exist for every possible phase of the solute and the solvent. In a solution, the particles of the substances are evenly distributed, resulting in a single phase. 1 or more substance that is. Solutions are homogeneous mixtures of two or more substances whose components are uniformly distributed on a microscopic scale. Homogeneous solutions are solutions with uniform composition and properties throughout the solution. A solution is a homogeneous mixture comprising smaller component/s called solute/s of small molecules or ions comparable in size to the molecules of a larger. Homogeneous mixtures, also known as solutions, are uniform throughout. For example a cup of. Trail mix, salad, and blood (which is also called a suspension) are examples of heterogenous mixtures. Single substance that does the dissolving substance present in the largest amount.
From answertion.com
How to solve for moment with uniform distributed load Q&A Answertion Uniform Solution Example Single substance that does the dissolving substance present in the largest amount. Trail mix, salad, and blood (which is also called a suspension) are examples of heterogenous mixtures. For example a cup of. Solutions are homogeneous mixtures of two or more substances whose components are uniformly distributed on a microscopic scale. Homogeneous solutions are solutions with uniform composition and properties. Uniform Solution Example.
From slideplayer.com
Solutions, Colloids, and Suspensions ppt download Uniform Solution Example Single substance that does the dissolving substance present in the largest amount. Solutions exist for every possible phase of the solute and the solvent. A solution is a homogeneous mixture comprising smaller component/s called solute/s of small molecules or ions comparable in size to the molecules of a larger. 1 or more substance that is. Solutions are homogeneous mixtures of. Uniform Solution Example.
From www.youtube.com
Uniform circular motion9th Class Physics YouTube Uniform Solution Example 1 or more substance that is. Single substance that does the dissolving substance present in the largest amount. Homogeneous mixtures, also known as solutions, are uniform throughout. In a solution, the particles of the substances are evenly distributed, resulting in a single phase. Salt water, for example, is a solution of solid \(\ce{nacl}\) in liquid water,. Trail mix, salad, and. Uniform Solution Example.
From www.researchgate.net
Example 5.1 the computed solution on an uniform mesh and an adapted Uniform Solution Example Trail mix, salad, and blood (which is also called a suspension) are examples of heterogenous mixtures. Homogeneous solutions are solutions with uniform composition and properties throughout the solution. Solutions exist for every possible phase of the solute and the solvent. Homogeneous mixtures, also known as solutions, are uniform throughout. Solutions are homogeneous mixtures of two or more substances whose components. Uniform Solution Example.
From www.researchgate.net
Example of a solutions space for í µí± uniform in the power Uniform Solution Example Trail mix, salad, and blood (which is also called a suspension) are examples of heterogenous mixtures. Salt water, for example, is a solution of solid \(\ce{nacl}\) in liquid water,. A solution is a homogeneous mixture comprising smaller component/s called solute/s of small molecules or ions comparable in size to the molecules of a larger. For example a cup of. Single. Uniform Solution Example.
From plainenglish.io
Uniform Cost Search (UCS) Algorithm in Python Uniform Solution Example Homogeneous mixtures, also known as solutions, are uniform throughout. Trail mix, salad, and blood (which is also called a suspension) are examples of heterogenous mixtures. In a solution, the particles of the substances are evenly distributed, resulting in a single phase. For example a cup of. Solutions exist for every possible phase of the solute and the solvent. Homogeneous solutions. Uniform Solution Example.
From www.studocu.com
Practice problem Continuous Uniform solution 41600 Practice Problems Uniform Solution Example In a solution, the particles of the substances are evenly distributed, resulting in a single phase. Trail mix, salad, and blood (which is also called a suspension) are examples of heterogenous mixtures. Homogeneous solutions are solutions with uniform composition and properties throughout the solution. A solution is a homogeneous mixture comprising smaller component/s called solute/s of small molecules or ions. Uniform Solution Example.
From sitedoct.org
Argumentative Essay About School Uniforms Should Be Required Uniform Solution Example For example a cup of. Single substance that does the dissolving substance present in the largest amount. Solutions exist for every possible phase of the solute and the solvent. Homogeneous mixtures, also known as solutions, are uniform throughout. Trail mix, salad, and blood (which is also called a suspension) are examples of heterogenous mixtures. Salt water, for example, is a. Uniform Solution Example.
From www.researchgate.net
Uniform and nonuniform solution sets on two types of planebased Uniform Solution Example Solutions exist for every possible phase of the solute and the solvent. Solutions are homogeneous mixtures of two or more substances whose components are uniformly distributed on a microscopic scale. A solution is a homogeneous mixture comprising smaller component/s called solute/s of small molecules or ions comparable in size to the molecules of a larger. Salt water, for example, is. Uniform Solution Example.
From www.slideserve.com
PPT Lecture (10) PowerPoint Presentation, free download ID6981311 Uniform Solution Example A solution is a homogeneous mixture comprising smaller component/s called solute/s of small molecules or ions comparable in size to the molecules of a larger. Single substance that does the dissolving substance present in the largest amount. Solutions exist for every possible phase of the solute and the solvent. Homogeneous solutions are solutions with uniform composition and properties throughout the. Uniform Solution Example.
From www.yourdictionary.com
Examples of Homogeneous Mixtures Solid, Liquid and Gas YourDictionary Uniform Solution Example Trail mix, salad, and blood (which is also called a suspension) are examples of heterogenous mixtures. Single substance that does the dissolving substance present in the largest amount. Homogeneous mixtures, also known as solutions, are uniform throughout. 1 or more substance that is. Homogeneous solutions are solutions with uniform composition and properties throughout the solution. Solutions are homogeneous mixtures of. Uniform Solution Example.
From www.thoughtco.com
10 Heterogeneous and Homogeneous Mixtures Uniform Solution Example A solution is a homogeneous mixture comprising smaller component/s called solute/s of small molecules or ions comparable in size to the molecules of a larger. Homogeneous mixtures, also known as solutions, are uniform throughout. For example a cup of. Salt water, for example, is a solution of solid \(\ce{nacl}\) in liquid water,. Solutions exist for every possible phase of the. Uniform Solution Example.
From www.slideserve.com
PPT CLASSIFICATION OF MATTER PowerPoint Presentation, free download Uniform Solution Example Homogeneous mixtures, also known as solutions, are uniform throughout. 1 or more substance that is. Single substance that does the dissolving substance present in the largest amount. A solution is a homogeneous mixture comprising smaller component/s called solute/s of small molecules or ions comparable in size to the molecules of a larger. Homogeneous solutions are solutions with uniform composition and. Uniform Solution Example.
From www.vrogue.co
What Is A Mixture Types Of Mixtures Chemistry Teachoo vrogue.co Uniform Solution Example Salt water, for example, is a solution of solid \(\ce{nacl}\) in liquid water,. Homogeneous mixtures, also known as solutions, are uniform throughout. 1 or more substance that is. For example a cup of. Solutions exist for every possible phase of the solute and the solvent. Single substance that does the dissolving substance present in the largest amount. Trail mix, salad,. Uniform Solution Example.
From chemistrytalk.org
What is a Solution in Chemistry? ChemTalk Uniform Solution Example For example a cup of. Single substance that does the dissolving substance present in the largest amount. Trail mix, salad, and blood (which is also called a suspension) are examples of heterogenous mixtures. A solution is a homogeneous mixture comprising smaller component/s called solute/s of small molecules or ions comparable in size to the molecules of a larger. Solutions exist. Uniform Solution Example.
From www.teachoo.com
Homogeneous and Hetrogeneous Mixtures Definition, Examples Teachoo Uniform Solution Example In a solution, the particles of the substances are evenly distributed, resulting in a single phase. Single substance that does the dissolving substance present in the largest amount. Homogeneous mixtures, also known as solutions, are uniform throughout. For example a cup of. Trail mix, salad, and blood (which is also called a suspension) are examples of heterogenous mixtures. Solutions exist. Uniform Solution Example.
From www.youtube.com
Continuous Probability Uniform Distribution Problems YouTube Uniform Solution Example Homogeneous mixtures, also known as solutions, are uniform throughout. A solution is a homogeneous mixture comprising smaller component/s called solute/s of small molecules or ions comparable in size to the molecules of a larger. Solutions are homogeneous mixtures of two or more substances whose components are uniformly distributed on a microscopic scale. Trail mix, salad, and blood (which is also. Uniform Solution Example.
From www.youtube.com
SOLUTIONS AND OTHER UNIFORM MIXTURES Science 6 by Sir C.G. YouTube Uniform Solution Example A solution is a homogeneous mixture comprising smaller component/s called solute/s of small molecules or ions comparable in size to the molecules of a larger. For example a cup of. Single substance that does the dissolving substance present in the largest amount. In a solution, the particles of the substances are evenly distributed, resulting in a single phase. Salt water,. Uniform Solution Example.
From www.numerade.com
What does it mean that a solution is “uniform thr… Uniform Solution Example Single substance that does the dissolving substance present in the largest amount. Homogeneous mixtures, also known as solutions, are uniform throughout. Salt water, for example, is a solution of solid \(\ce{nacl}\) in liquid water,. Trail mix, salad, and blood (which is also called a suspension) are examples of heterogenous mixtures. Homogeneous solutions are solutions with uniform composition and properties throughout. Uniform Solution Example.
From examples.yourdictionary.com
Examples of Heterogeneous Mixtures Types Made Simple YourDictionary Uniform Solution Example Single substance that does the dissolving substance present in the largest amount. A solution is a homogeneous mixture comprising smaller component/s called solute/s of small molecules or ions comparable in size to the molecules of a larger. Trail mix, salad, and blood (which is also called a suspension) are examples of heterogenous mixtures. Solutions exist for every possible phase of. Uniform Solution Example.
From www.chegg.com
Solved 5. Mixture of Uniforms Bookmark this page Homework Uniform Solution Example For example a cup of. 1 or more substance that is. In a solution, the particles of the substances are evenly distributed, resulting in a single phase. Single substance that does the dissolving substance present in the largest amount. Solutions are homogeneous mixtures of two or more substances whose components are uniformly distributed on a microscopic scale. Salt water, for. Uniform Solution Example.
From mavink.com
Uniform Cost Search Algorithm Uniform Solution Example Homogeneous mixtures, also known as solutions, are uniform throughout. 1 or more substance that is. Trail mix, salad, and blood (which is also called a suspension) are examples of heterogenous mixtures. Salt water, for example, is a solution of solid \(\ce{nacl}\) in liquid water,. Solutions exist for every possible phase of the solute and the solvent. A solution is a. Uniform Solution Example.
From www.youtube.com
Uniform Distribution EXPLAINED with Examples YouTube Uniform Solution Example A solution is a homogeneous mixture comprising smaller component/s called solute/s of small molecules or ions comparable in size to the molecules of a larger. Homogeneous solutions are solutions with uniform composition and properties throughout the solution. Trail mix, salad, and blood (which is also called a suspension) are examples of heterogenous mixtures. In a solution, the particles of the. Uniform Solution Example.
From www.youtube.com
(Three moment equation) Simply supported beam with uniformly Uniform Solution Example In a solution, the particles of the substances are evenly distributed, resulting in a single phase. Solutions are homogeneous mixtures of two or more substances whose components are uniformly distributed on a microscopic scale. Homogeneous solutions are solutions with uniform composition and properties throughout the solution. For example a cup of. Solutions exist for every possible phase of the solute. Uniform Solution Example.
From www.youtube.com
Distributed loading on a beam example 1 rectangular loads YouTube Uniform Solution Example Trail mix, salad, and blood (which is also called a suspension) are examples of heterogenous mixtures. Salt water, for example, is a solution of solid \(\ce{nacl}\) in liquid water,. 1 or more substance that is. A solution is a homogeneous mixture comprising smaller component/s called solute/s of small molecules or ions comparable in size to the molecules of a larger.. Uniform Solution Example.
From www.slideserve.com
PPT CH110 Chapter 8 Solutions PowerPoint Presentation, free download Uniform Solution Example For example a cup of. Trail mix, salad, and blood (which is also called a suspension) are examples of heterogenous mixtures. A solution is a homogeneous mixture comprising smaller component/s called solute/s of small molecules or ions comparable in size to the molecules of a larger. Homogeneous mixtures, also known as solutions, are uniform throughout. Solutions are homogeneous mixtures of. Uniform Solution Example.
From sciencenotes.org
What Is a Homogeneous Mixture? Definition and Examples Uniform Solution Example 1 or more substance that is. Solutions are homogeneous mixtures of two or more substances whose components are uniformly distributed on a microscopic scale. A solution is a homogeneous mixture comprising smaller component/s called solute/s of small molecules or ions comparable in size to the molecules of a larger. Homogeneous solutions are solutions with uniform composition and properties throughout the. Uniform Solution Example.
From www.expii.com
What Are Mixtures — Definition & Overview Expii Uniform Solution Example Salt water, for example, is a solution of solid \(\ce{nacl}\) in liquid water,. Homogeneous solutions are solutions with uniform composition and properties throughout the solution. Single substance that does the dissolving substance present in the largest amount. In a solution, the particles of the substances are evenly distributed, resulting in a single phase. For example a cup of. Trail mix,. Uniform Solution Example.
From www.parthapakray.com
Example I Uniform Solution Example Homogeneous mixtures, also known as solutions, are uniform throughout. Single substance that does the dissolving substance present in the largest amount. Homogeneous solutions are solutions with uniform composition and properties throughout the solution. In a solution, the particles of the substances are evenly distributed, resulting in a single phase. For example a cup of. Trail mix, salad, and blood (which. Uniform Solution Example.
From www.len.com.ng
Types of Mixture Homogenous and Heterogeneous Mixtures Uniform Solution Example Salt water, for example, is a solution of solid \(\ce{nacl}\) in liquid water,. In a solution, the particles of the substances are evenly distributed, resulting in a single phase. Homogeneous mixtures, also known as solutions, are uniform throughout. Solutions are homogeneous mixtures of two or more substances whose components are uniformly distributed on a microscopic scale. Solutions exist for every. Uniform Solution Example.
From www.vrogue.co
Cinder Block Retaining Wall Height Cinder Block Retai vrogue.co Uniform Solution Example Solutions are homogeneous mixtures of two or more substances whose components are uniformly distributed on a microscopic scale. A solution is a homogeneous mixture comprising smaller component/s called solute/s of small molecules or ions comparable in size to the molecules of a larger. For example a cup of. In a solution, the particles of the substances are evenly distributed, resulting. Uniform Solution Example.
From www.publicdomainpictures.net
Second World War, Military Uniform Free Stock Photo Public Domain Uniform Solution Example For example a cup of. Salt water, for example, is a solution of solid \(\ce{nacl}\) in liquid water,. Solutions are homogeneous mixtures of two or more substances whose components are uniformly distributed on a microscopic scale. 1 or more substance that is. Solutions exist for every possible phase of the solute and the solvent. A solution is a homogeneous mixture. Uniform Solution Example.
From www.slideserve.com
PPT Statistical Distributions PowerPoint Presentation, free download Uniform Solution Example In a solution, the particles of the substances are evenly distributed, resulting in a single phase. Solutions are homogeneous mixtures of two or more substances whose components are uniformly distributed on a microscopic scale. Homogeneous mixtures, also known as solutions, are uniform throughout. Salt water, for example, is a solution of solid \(\ce{nacl}\) in liquid water,. A solution is a. Uniform Solution Example.
From analystprep.com
Continuous Unifrom Distribution Example CFA Level 1 AnalystPrep Uniform Solution Example Homogeneous mixtures, also known as solutions, are uniform throughout. For example a cup of. 1 or more substance that is. Solutions are homogeneous mixtures of two or more substances whose components are uniformly distributed on a microscopic scale. A solution is a homogeneous mixture comprising smaller component/s called solute/s of small molecules or ions comparable in size to the molecules. Uniform Solution Example.
From calcworkshop.com
Continuous Uniform Distribution (Defined w/ 5 Examples!) Uniform Solution Example A solution is a homogeneous mixture comprising smaller component/s called solute/s of small molecules or ions comparable in size to the molecules of a larger. 1 or more substance that is. Salt water, for example, is a solution of solid \(\ce{nacl}\) in liquid water,. In a solution, the particles of the substances are evenly distributed, resulting in a single phase.. Uniform Solution Example.