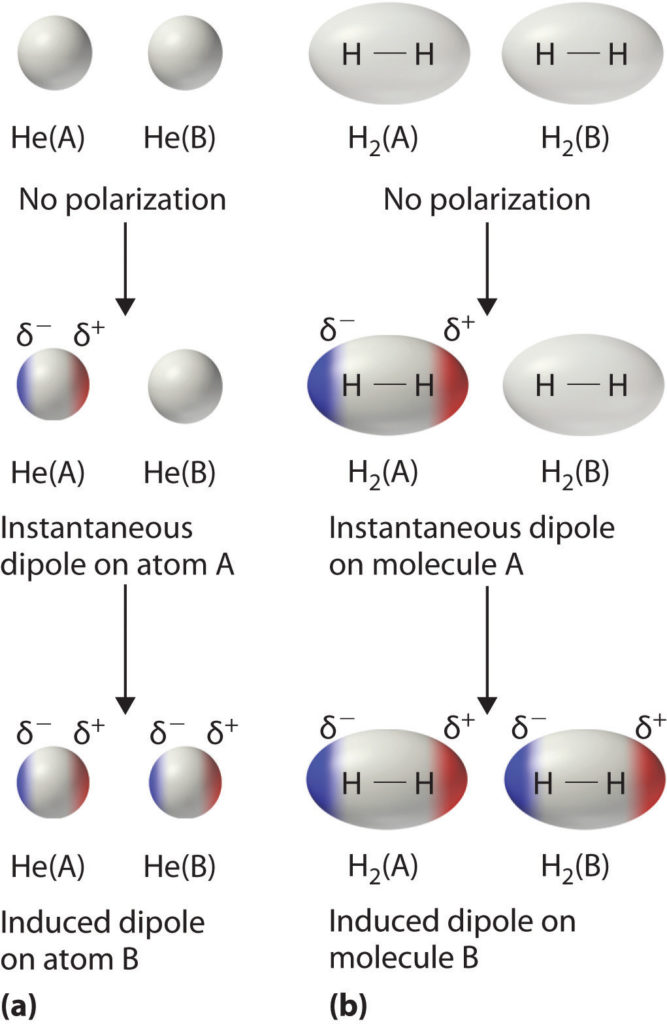
What Do Van Der Waals Forces Do . Van der waals forces are specific intermolecular interactions observed in liquids and solids. For example, van der waals forces can arise from the fluctuation in the polarizations of two particles that are close to. Van der waals forces' is a general term used to define the attraction of intermolecular forces between molecules. What are van der waals forces? Covalent bonds are strong intramolecular forces. Molecules also contain weaker intermolecular forces which are forces between molecules. Van der waals' forces & dipoles. There are two kinds of van. Van der waals forces are weak intermolecular forces that are dependent on the distance between atoms or molecules. These forces arise from the interactions between uncharged atoms/molecules. They are electrostatic in nature, arising from the interactions of positively and negatively. Van der waals forces, relatively weak electric forces that attract neutral molecules to one another in gases, in liquefied and solidified gases, and in almost all.
from www.crediblehulk.org
What are van der waals forces? They are electrostatic in nature, arising from the interactions of positively and negatively. For example, van der waals forces can arise from the fluctuation in the polarizations of two particles that are close to. Van der waals forces' is a general term used to define the attraction of intermolecular forces between molecules. Van der waals forces are weak intermolecular forces that are dependent on the distance between atoms or molecules. Van der waals forces, relatively weak electric forces that attract neutral molecules to one another in gases, in liquefied and solidified gases, and in almost all. Covalent bonds are strong intramolecular forces. There are two kinds of van. Van der waals' forces & dipoles. Van der waals forces are specific intermolecular interactions observed in liquids and solids.
Van der Waals (London Dispersion) Forces The Credible Hulk
What Do Van Der Waals Forces Do Van der waals forces' is a general term used to define the attraction of intermolecular forces between molecules. There are two kinds of van. They are electrostatic in nature, arising from the interactions of positively and negatively. These forces arise from the interactions between uncharged atoms/molecules. Covalent bonds are strong intramolecular forces. Van der waals forces are specific intermolecular interactions observed in liquids and solids. For example, van der waals forces can arise from the fluctuation in the polarizations of two particles that are close to. Van der waals forces, relatively weak electric forces that attract neutral molecules to one another in gases, in liquefied and solidified gases, and in almost all. What are van der waals forces? Van der waals' forces & dipoles. Molecules also contain weaker intermolecular forces which are forces between molecules. Van der waals forces are weak intermolecular forces that are dependent on the distance between atoms or molecules. Van der waals forces' is a general term used to define the attraction of intermolecular forces between molecules.
From ar.inspiredpencil.com
Van Der Waals Forces Definition What Do Van Der Waals Forces Do There are two kinds of van. Van der waals forces' is a general term used to define the attraction of intermolecular forces between molecules. For example, van der waals forces can arise from the fluctuation in the polarizations of two particles that are close to. Molecules also contain weaker intermolecular forces which are forces between molecules. Van der waals forces. What Do Van Der Waals Forces Do.
From www.slideserve.com
PPT Bonding PowerPoint Presentation, free download ID5339686 What Do Van Der Waals Forces Do They are electrostatic in nature, arising from the interactions of positively and negatively. Covalent bonds are strong intramolecular forces. Van der waals forces' is a general term used to define the attraction of intermolecular forces between molecules. What are van der waals forces? Van der waals forces, relatively weak electric forces that attract neutral molecules to one another in gases,. What Do Van Der Waals Forces Do.
From www.slideserve.com
PPT Van der Waals Forces PowerPoint Presentation, free download ID What Do Van Der Waals Forces Do What are van der waals forces? Van der waals forces' is a general term used to define the attraction of intermolecular forces between molecules. Van der waals' forces & dipoles. Van der waals forces are weak intermolecular forces that are dependent on the distance between atoms or molecules. These forces arise from the interactions between uncharged atoms/molecules. Covalent bonds are. What Do Van Der Waals Forces Do.
From mavink.com
What Is Van Der Waals Force What Do Van Der Waals Forces Do There are two kinds of van. Van der waals' forces & dipoles. Van der waals forces' is a general term used to define the attraction of intermolecular forces between molecules. They are electrostatic in nature, arising from the interactions of positively and negatively. Van der waals forces are weak intermolecular forces that are dependent on the distance between atoms or. What Do Van Der Waals Forces Do.
From byjus.com
Van Der Waals Forces (Intermolecular Bonding) Definition & Examples What Do Van Der Waals Forces Do Van der waals' forces & dipoles. There are two kinds of van. Van der waals forces, relatively weak electric forces that attract neutral molecules to one another in gases, in liquefied and solidified gases, and in almost all. They are electrostatic in nature, arising from the interactions of positively and negatively. Van der waals forces are specific intermolecular interactions observed. What Do Van Der Waals Forces Do.
From www.slideserve.com
PPT Van der Waals Forces PowerPoint Presentation, free download ID What Do Van Der Waals Forces Do For example, van der waals forces can arise from the fluctuation in the polarizations of two particles that are close to. Van der waals' forces & dipoles. There are two kinds of van. They are electrostatic in nature, arising from the interactions of positively and negatively. These forces arise from the interactions between uncharged atoms/molecules. Molecules also contain weaker intermolecular. What Do Van Der Waals Forces Do.
From www.pinterest.com
Van Der Waals Force Easy Science Easy science, Force definition What Do Van Der Waals Forces Do What are van der waals forces? There are two kinds of van. For example, van der waals forces can arise from the fluctuation in the polarizations of two particles that are close to. Molecules also contain weaker intermolecular forces which are forces between molecules. Van der waals forces are weak intermolecular forces that are dependent on the distance between atoms. What Do Van Der Waals Forces Do.
From www.youtube.com
Van Der Waals Forces YouTube What Do Van Der Waals Forces Do Van der waals forces, relatively weak electric forces that attract neutral molecules to one another in gases, in liquefied and solidified gases, and in almost all. They are electrostatic in nature, arising from the interactions of positively and negatively. Molecules also contain weaker intermolecular forces which are forces between molecules. These forces arise from the interactions between uncharged atoms/molecules. Van. What Do Van Der Waals Forces Do.
From www.science-revision.co.uk
Van der Waals bonding What Do Van Der Waals Forces Do These forces arise from the interactions between uncharged atoms/molecules. What are van der waals forces? Covalent bonds are strong intramolecular forces. Molecules also contain weaker intermolecular forces which are forces between molecules. There are two kinds of van. They are electrostatic in nature, arising from the interactions of positively and negatively. For example, van der waals forces can arise from. What Do Van Der Waals Forces Do.
From www.slideserve.com
PPT Summary on intermolecular forces PowerPoint Presentation ID432746 What Do Van Der Waals Forces Do Van der waals forces' is a general term used to define the attraction of intermolecular forces between molecules. For example, van der waals forces can arise from the fluctuation in the polarizations of two particles that are close to. Van der waals' forces & dipoles. Covalent bonds are strong intramolecular forces. Molecules also contain weaker intermolecular forces which are forces. What Do Van Der Waals Forces Do.
From www.slideserve.com
PPT Intermolecular Forces a.k.a. van der Waal’s Forces PowerPoint What Do Van Der Waals Forces Do Van der waals forces, relatively weak electric forces that attract neutral molecules to one another in gases, in liquefied and solidified gases, and in almost all. Van der waals forces are specific intermolecular interactions observed in liquids and solids. For example, van der waals forces can arise from the fluctuation in the polarizations of two particles that are close to.. What Do Van Der Waals Forces Do.
From www.youtube.com
What are Vander Waals Forces? Types of Wander Waals Forces l London What Do Van Der Waals Forces Do Van der waals' forces & dipoles. Van der waals forces, relatively weak electric forces that attract neutral molecules to one another in gases, in liquefied and solidified gases, and in almost all. Van der waals forces are weak intermolecular forces that are dependent on the distance between atoms or molecules. Van der waals forces are specific intermolecular interactions observed in. What Do Van Der Waals Forces Do.
From vanderwaals.weebly.com
Description Van der Waals Intermolecular forces What Do Van Der Waals Forces Do Van der waals forces, relatively weak electric forces that attract neutral molecules to one another in gases, in liquefied and solidified gases, and in almost all. For example, van der waals forces can arise from the fluctuation in the polarizations of two particles that are close to. Van der waals forces are weak intermolecular forces that are dependent on the. What Do Van Der Waals Forces Do.
From www.expii.com
Van der Waals Forces — Definition & Overview Expii What Do Van Der Waals Forces Do These forces arise from the interactions between uncharged atoms/molecules. Molecules also contain weaker intermolecular forces which are forces between molecules. Van der waals forces are weak intermolecular forces that are dependent on the distance between atoms or molecules. Van der waals' forces & dipoles. They are electrostatic in nature, arising from the interactions of positively and negatively. What are van. What Do Van Der Waals Forces Do.
From www.youtube.com
INTERMOLECULAR FORCES VAN DER WAALS, DIPOLEDIPOLE INTERACTION AND What Do Van Der Waals Forces Do For example, van der waals forces can arise from the fluctuation in the polarizations of two particles that are close to. Van der waals forces' is a general term used to define the attraction of intermolecular forces between molecules. There are two kinds of van. Van der waals' forces & dipoles. Van der waals forces are weak intermolecular forces that. What Do Van Der Waals Forces Do.
From chemistrytalk.org
London Dispersion and Van der Waals Forces ChemTalk What Do Van Der Waals Forces Do For example, van der waals forces can arise from the fluctuation in the polarizations of two particles that are close to. Van der waals' forces & dipoles. Covalent bonds are strong intramolecular forces. What are van der waals forces? There are two kinds of van. Van der waals forces, relatively weak electric forces that attract neutral molecules to one another. What Do Van Der Waals Forces Do.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID What Do Van Der Waals Forces Do There are two kinds of van. Molecules also contain weaker intermolecular forces which are forces between molecules. Covalent bonds are strong intramolecular forces. For example, van der waals forces can arise from the fluctuation in the polarizations of two particles that are close to. Van der waals forces, relatively weak electric forces that attract neutral molecules to one another in. What Do Van Der Waals Forces Do.
From www.slideserve.com
PPT Van der Waals Forces PowerPoint Presentation, free download ID What Do Van Der Waals Forces Do These forces arise from the interactions between uncharged atoms/molecules. What are van der waals forces? Van der waals forces' is a general term used to define the attraction of intermolecular forces between molecules. Covalent bonds are strong intramolecular forces. Van der waals forces are specific intermolecular interactions observed in liquids and solids. There are two kinds of van. Van der. What Do Van Der Waals Forces Do.
From www.pinterest.com
How do hydrogen bonds form? How do they compare to Van der Waals Forces What Do Van Der Waals Forces Do Molecules also contain weaker intermolecular forces which are forces between molecules. These forces arise from the interactions between uncharged atoms/molecules. Covalent bonds are strong intramolecular forces. There are two kinds of van. Van der waals forces, relatively weak electric forces that attract neutral molecules to one another in gases, in liquefied and solidified gases, and in almost all. For example,. What Do Van Der Waals Forces Do.
From mavink.com
What Is Van Der Waals Force What Do Van Der Waals Forces Do Van der waals forces, relatively weak electric forces that attract neutral molecules to one another in gases, in liquefied and solidified gases, and in almost all. Van der waals forces are weak intermolecular forces that are dependent on the distance between atoms or molecules. They are electrostatic in nature, arising from the interactions of positively and negatively. These forces arise. What Do Van Der Waals Forces Do.
From www.chemistrylearner.com
Van der Waals Forces (Bond) Definition, Examples, & Diagrams What Do Van Der Waals Forces Do Van der waals' forces & dipoles. Van der waals forces, relatively weak electric forces that attract neutral molecules to one another in gases, in liquefied and solidified gases, and in almost all. These forces arise from the interactions between uncharged atoms/molecules. Van der waals forces are weak intermolecular forces that are dependent on the distance between atoms or molecules. Covalent. What Do Van Der Waals Forces Do.
From www.crediblehulk.org
Van der Waals (London Dispersion) Forces The Credible Hulk What Do Van Der Waals Forces Do Molecules also contain weaker intermolecular forces which are forces between molecules. For example, van der waals forces can arise from the fluctuation in the polarizations of two particles that are close to. Van der waals forces are specific intermolecular interactions observed in liquids and solids. Van der waals forces, relatively weak electric forces that attract neutral molecules to one another. What Do Van Der Waals Forces Do.
From www.congress-intercultural.eu
Debye Explains The Mechanism Of Van Der Waals Forces, 57 OFF What Do Van Der Waals Forces Do Covalent bonds are strong intramolecular forces. They are electrostatic in nature, arising from the interactions of positively and negatively. There are two kinds of van. For example, van der waals forces can arise from the fluctuation in the polarizations of two particles that are close to. Van der waals forces' is a general term used to define the attraction of. What Do Van Der Waals Forces Do.
From www.youtube.com
Ordering relative strength of Van der Waals Force compared to other What Do Van Der Waals Forces Do For example, van der waals forces can arise from the fluctuation in the polarizations of two particles that are close to. Van der waals forces are specific intermolecular interactions observed in liquids and solids. Molecules also contain weaker intermolecular forces which are forces between molecules. There are two kinds of van. What are van der waals forces? Covalent bonds are. What Do Van Der Waals Forces Do.
From tipseri.com
What do van der Waals forces mean? Tipseri What Do Van Der Waals Forces Do Van der waals forces are specific intermolecular interactions observed in liquids and solids. These forces arise from the interactions between uncharged atoms/molecules. Van der waals forces are weak intermolecular forces that are dependent on the distance between atoms or molecules. Molecules also contain weaker intermolecular forces which are forces between molecules. Covalent bonds are strong intramolecular forces. Van der waals. What Do Van Der Waals Forces Do.
From www.chemistrylearner.com
Van der Waals Forces (Bond) Definition, Examples, & Diagrams What Do Van Der Waals Forces Do Van der waals' forces & dipoles. Van der waals forces' is a general term used to define the attraction of intermolecular forces between molecules. Molecules also contain weaker intermolecular forces which are forces between molecules. Van der waals forces, relatively weak electric forces that attract neutral molecules to one another in gases, in liquefied and solidified gases, and in almost. What Do Van Der Waals Forces Do.
From www.electricity-magnetism.org
What are Van der Waals forces? What Do Van Der Waals Forces Do Van der waals' forces & dipoles. There are two kinds of van. Covalent bonds are strong intramolecular forces. Van der waals forces, relatively weak electric forces that attract neutral molecules to one another in gases, in liquefied and solidified gases, and in almost all. They are electrostatic in nature, arising from the interactions of positively and negatively. Van der waals. What Do Van Der Waals Forces Do.
From www.slideserve.com
PPT Topic 4 Bonding . SL + HL PowerPoint Presentation, free download What Do Van Der Waals Forces Do Van der waals forces, relatively weak electric forces that attract neutral molecules to one another in gases, in liquefied and solidified gases, and in almost all. Covalent bonds are strong intramolecular forces. For example, van der waals forces can arise from the fluctuation in the polarizations of two particles that are close to. Van der waals forces are specific intermolecular. What Do Van Der Waals Forces Do.
From www.slideserve.com
PPT Chapter 2 Representative Carbon Compounds Functional Groups What Do Van Der Waals Forces Do For example, van der waals forces can arise from the fluctuation in the polarizations of two particles that are close to. Van der waals forces are specific intermolecular interactions observed in liquids and solids. Van der waals forces' is a general term used to define the attraction of intermolecular forces between molecules. They are electrostatic in nature, arising from the. What Do Van Der Waals Forces Do.
From www.slideserve.com
PPT Covalent Compounds PowerPoint Presentation, free download ID What Do Van Der Waals Forces Do Van der waals forces' is a general term used to define the attraction of intermolecular forces between molecules. Molecules also contain weaker intermolecular forces which are forces between molecules. Covalent bonds are strong intramolecular forces. Van der waals' forces & dipoles. These forces arise from the interactions between uncharged atoms/molecules. What are van der waals forces? Van der waals forces. What Do Van Der Waals Forces Do.
From ar.inspiredpencil.com
Van Der Waals Forces Example What Do Van Der Waals Forces Do There are two kinds of van. Van der waals' forces & dipoles. What are van der waals forces? Van der waals forces, relatively weak electric forces that attract neutral molecules to one another in gases, in liquefied and solidified gases, and in almost all. They are electrostatic in nature, arising from the interactions of positively and negatively. Van der waals. What Do Van Der Waals Forces Do.
From sytech.co.zw
What Are Van Der Waals' Forces? What Do Van Der Waals Forces Do Van der waals forces are weak intermolecular forces that are dependent on the distance between atoms or molecules. Van der waals forces' is a general term used to define the attraction of intermolecular forces between molecules. Molecules also contain weaker intermolecular forces which are forces between molecules. Van der waals forces are specific intermolecular interactions observed in liquids and solids.. What Do Van Der Waals Forces Do.
From socratic.org
Why do Van der Waals forces increase with the size of molecules? Socratic What Do Van Der Waals Forces Do Van der waals forces are weak intermolecular forces that are dependent on the distance between atoms or molecules. These forces arise from the interactions between uncharged atoms/molecules. Molecules also contain weaker intermolecular forces which are forces between molecules. They are electrostatic in nature, arising from the interactions of positively and negatively. Van der waals forces, relatively weak electric forces that. What Do Van Der Waals Forces Do.
From www.vecteezy.com
Van der waals force is a distancedependent interaction between atoms What Do Van Der Waals Forces Do Molecules also contain weaker intermolecular forces which are forces between molecules. Van der waals forces, relatively weak electric forces that attract neutral molecules to one another in gases, in liquefied and solidified gases, and in almost all. Covalent bonds are strong intramolecular forces. They are electrostatic in nature, arising from the interactions of positively and negatively. There are two kinds. What Do Van Der Waals Forces Do.
From www.myxxgirl.com
How Do Van Der Waals Forces Hold Molecules Together Sciencing My XXX What Do Van Der Waals Forces Do Van der waals forces are specific intermolecular interactions observed in liquids and solids. These forces arise from the interactions between uncharged atoms/molecules. Van der waals forces, relatively weak electric forces that attract neutral molecules to one another in gases, in liquefied and solidified gases, and in almost all. What are van der waals forces? There are two kinds of van.. What Do Van Der Waals Forces Do.